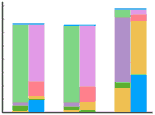
Unit VII-Excretion
Chapter 23
Regulation of the Extracellular Fluid by the Kidneys
1. General Remarks:
It was pointed out in Chapter 9 that cells are quite different in composition from the interstitium in which they are imbedded. The interstitium together with the plasma makes up a cellular environment whose composition is quite critical for the function of most cells. For example, cells of the central nervous system cannot function when the plasma and interstitium (often referred to together as the extracellular fluid) are abnormally acid or alkaline. Similarly calcium, magnesium, and potassium concentrations in the extracellular fluid must be regulated within fairly narrow limits.
The idea that constancy of the cellular or internal environment is essential for normal function was first stated by the French physiologist Claude Bernard. It has been speculated since then that the internal cellular environment is a fluid similar to the sea water in which life arose, and that the animals of today carry a little of that ancient sea around with them. The evidence in favor of this constantly repeated set of propositions is remarkably weak, but it is no longer quite respectable to doubt any part of the implied scheme, at least in intellectual circles.
The composition of the extracellular fluid reflects less what the animal eats and drinks than what the excretory organs leave behind. Thus, using an example already familiar, it is virtually impossible for an animal to lower the osmolarity of its body fluids by drinking water--the excess of water taken is simply discarded by the kidneys. An animal eating foods whose metabolism leads to the production of strong acids can easily keep its extracellular hydrogen ion concentration nearly constant by increasing its pulmonary output of carbon dioxide. This will be considered in more detail in the next parts of this chapter.
2. Acidity of the Body Fluids:
The acidity of a solution can be described by the concentration of free hydrogen ion in the solution. The higher the concentration of this ion the more acid is the solution. Undissociated hydrogen does not contribute to the acidity of a solution; hydrogen strongly (but not entirely) bound by another ion contributes little. Thus, the highly dissociated hydrogen ion of hydrochloric acid (H+Cl-) can make a strong acid; the weakly dissociated hydrogen ion of carbonic acid HCO3 makes at best, a rather weak acid and the almost completely undissociated hydrogen of sodium bicarbonate NaHCO3 contributes to acidity not at all.
The range of hydrogen ion concentrations in the extracellular fluid is very small. The normal value is 0.00000004 N. A rise to 0.00000010 N is a very severe acidosis, and a fall to 0.00000002 N is a dangerous alkalosis. These concentrations are inconveniently small to write and to simplify discussion of hydrogen ion concentration it is customary to use a unit called pH. This unit is simply the negative logarithm to the base 10 of the hydrogen ion concentration. For example, a 10 N solution of hydrochloric acid yielding a hydrogen ion concentration of 10 equivalents per liter is said to have a pH of -1. (The logarithm of 10 is 1; by changing the sign of the logarithm we arrive at the pH -1). A solution of 0.1 N hydrochloric acid has a pH of +1. (The logarithm of 0.1 is -1; by changing the sign, we arrive at the pH = +1). Pure water has a hydrogen ion concentration of 0.0030001 N. The pH is 7. (The logarithm of 0.0003031 is -7; by changing the sign, we arrive at the pH = +7.) Even strongly alkaline solutions contain some H+ ion. For example, if 1.0 N NaOH contains H+ ion at a concentration of 10-15, such a solution has a pH of 15.
The student who is uneasy with logarithms may be helped in the following by a set of simple rules. Acid solutions have pH less than 7; neutral solutions have a pH of 7. Alkaline solutions have pH more than 7. The higher the pH, the lower the hydrogen ion concentration. Thus, the statement that the pH of extracellular fluid is normally 7.4 can be interpreted to mean that this fluid is slightly less acid than water, or a little alkaline. A solution whose pH is 8.0 is only a little more alkaline than one with a pH of 7.4; but the cells of the central nervous system are so sensitive to changes in hydrogen ion concentration that they cannot survive in such a fluid. Death occurs in alkalosis. Although a pH of 7.0 is neutral, it is intolerably acid compared to the normal value of 7.4. When the extracellular fluid pH falls to 7.0 death occurs in acidosis.
3. Buffers:
As indicatad above, the body does not tolerate even very small changes in hydrogen ion concentration in the extracellular fluids. Yet in daily life, these fluids are constantly challenged by large movements of hydrogen ions in either direction. For example, large quantities of strong acids are produced in anerobic muscular exercise and released into the blood. In the other direction, large quantities of hydrogen ions are removed from the blood during the secretion of hydrochloric acid by the stomach. Why then is muscular exercise not associated with an immediate acidosis? Why is not hydrochloric acid secretion associated with intolerable alkalosis?
The answer has to do with the fact that the body fluids are very well buffered to resist pH changes. (The word buffer is used to describe something which deadens a shock or a buffet). Part of the buffering is chemical-part is physiological. Sudden changes in hydrogen ions are buffered chemically at first, and by the action of the lungs and kidneys very shortly afterwards.
Let us begin by considering chemical buffering: suppose that we have a mixture of two substances one of which is capable of reaction with added hydrogen ion (binding it); while the other is capable of releasing hydrogen ions removed from solution. Such a mixture might be made from disodium and monosodium phosphates as follows:
Na22+HPO42- / Na+H2PO4-
If a hydrogen ion is added to the above mixture, it will be bound by the HPO42- ion, which now becomes H2PO4-, so that the hydrogen ion does not appear by itself.
H+ + HPO42- --> H2PO4-
Similarly, a hydrogen ion can be removed from this mixture by the addition of OH- ion; but there will be little change of hydrogen ion concentration because of the reaction between H2PO4- and OH- to form water and HPO42-. This mixture then can be attacked from either side, by hydrogen ions or by OH- ions with little overall change in hydrogen ion concentration.
OH- + H2PO4- --> H2O + HPO42-
The perceptive student may by now have noted that something seems to be wrong here. The HPO42- is supposed to bind hydrogen ions, forming H2PO4-, the hydrogen being absorbed without a trace. Yet H2PO4- is also supposed to be able to supply hydrogen ion to neutralize added OH-.
Actually, there is no real paradox. HPO42- does indeed bind hydrogen ions, though not quite without a trace; a little dissociates. When OH- is added to H2PO4-, the little H+ which dissociates is "captured" by the OH- and more dissociates in accordance with the laws of chemical equilibrium.
The same type of behavior is shown by the buffer system:
H22+CO32- /
Na+H+CO32-
( Carbonic acid / Sodium bicarbonate )
The carbonic acid is a weak acid, though a little H+ is available from it.
If hydrogen ion is added to the above system it reacts with HCO3- to produce more H2CO3. Most of the added hydrogen ions are bound, and the attack on the pH of the system is blunted. Conversely, if OH- is added, it reacts with the small amounts of H+ available from H2CO3, forming H2O and HCO3-. Again, the edge is taken off the attack on the pH of the system. The system is protected against wide swings in pH produced by the addition of either acid or alkali.
H+ + HCO3- --> H2CO3
OH- + H2CO3 --> H2O + HCO3-
The protection is not complete, as shown by the following equation. This equation, called the Henderson-Hasselbach equation, will not be derived here, but it will be employed in the next part of this chapter and the student should attempt to understand what it says.
The pH of a buffer mixture can be written in terms of a constant characteristic of that mixture and the ratio of the concentration of its alkaline member to its acid member as follows:
| pH = pKa + log ( | Concentration of salt of weak acid | ) |
| Concentration of weak acid |
Here the Henderson-Hasselbach equation has been written for the bicarbonate-carbonic acid mixture. The characteristic constant for this mixture is 6.1:
| pH = 6.1 + log ( | NaHCO3 | ) |
| H2CO3 |
If the concentration of NaHCO3 and H2CO3 are 24 mEq/L and 1.2 mEq/L, respectively, the pH of the mixture is:
| pH = 6.1 + log ( | 24 | ) |
| 1.2 |
pH = 6.1 + log 20
pH = 6.1 + 1.3 = 7.4
Suppose that 9 mEq of HCl is added to a liter of this solution. This will react with 9 mEq of the NaHCO3, lowering its concentration to 15 mEq/L. If the carbonic acid newly formed remained in solution (it does not), the pH of the mixture would fall to 6.1. If the carbonic acid is allowed to escape from solution, breaking up into H2O and CO2, the concentration of H2CO3 will remain as it was, 1.2 mEq/L, and the pH will be 7.2.
If we consider that 3.3 mEq of HCl in a liter of water would reduce its pH to about 1.88, and that the buffer system keeps the change down to 0.2 pH units, the stability of the pH of the extra-cellular fluid becomes easy to understand.
The bicarbonate-carbonic acid buffer system accounts for about half the immediate buffering capacity of the body. Most of the remainder is associated with the buffer properties of proteins, whether intracellular or extracellular. These act as buffers by virtue of the fact that they have amino groups and carboxyl which react with H+ ions in acid solutions and give up H+ ions in alkaline solutions. Though these buffers serve to protect from very sudden changes in pH, their original status must be restored by biological buffering.
4. Biological Buffering:
The chemical buffers described in the last section are useful in protecting the animal from the pH changes to be expected from sudden production or withdrawal of hydrogen ions. The buffers absorb the shock. However, in absorbing the shock, the buffer systems are in part used up. In order to maintain the status quo, the organism must reconstitute the buffer system. Basically, this is the business of the lungs and the kidneys. The lungs act by excreting more or less carbon dioxide; the kidneys act by eliminating hydrogen ions (or ammonium ions) along with the acid anion which produced acidosis, or by eliminating bicarbonate ion when alkalosis has been produced. These statements may become clearer when examples are given.
Assume that in the course of metabolism, a strong acid is generated and released into the blood stream. The buffer systems will react with this acid and most of the invasion by the hydrogen ions will be countered by them. We will concentrate here on the bicarbonate-carbonic acid system. The strong acid, reacting with bicarbonate, produces carbonic acid, which is a respiratory stimulant both as an acid and as CO2. Bicarbonate falls, H+ rises a little and CO2 also rises; increased respiratory activity follows and in a very short time, the normal pH of the body may be restored. The correction is, however, a faulty one. Added acid shows up as diminished bicarbonate and diminished CO2. The ratio between these may be quite normal, so that the pH is normal. Nevertheless, the correction in pH has been purchased through depletion of the whole bicarbonate-carbonic acid buffer system.
In order to achieve restitution of this system, the kidneys must eliminate the strong acid which provoked the original difficulty, either directly or as a potassium or ammonium salt and replace the anion with bicarbonate; the lungs must simultaneously retain a little more CO2 than is produced.
Figure 325 shows the events as occuring in five phases. In the first phase, the extracellular fluid comes under attack by metabolic acid. This acid is chemically buffered in phase 2 and biologically buffered by the lungs in phase 3. Restitution of the normal bicarbonate and carbonic acid occurs through the corrective actions of the kidney (phase 4) and lungs (phase 5).
Note that in the example chosen, a metabolic acid is finally excreted and the buffering systems of the body are restored.
The loss of hydrogen ions from the extracellular fluid as in the production of gastric hydrochloric acid is reflected in the bicarbonate-carbonic acid buffer system as an increase in bicarbonate. The kidney corrects this easily by excreting bicarbonate whenever its concentration rises above 28 mEq/L. The behavior of the kidney toward this ion is not fully understood, but it does appear that all bicarbonate is reahsorbed from the glomerular filtrate when its concentration is less than 28 mEq/L. At higher concentrations, the mechanisms reabsorbing bicarbonate appear to be saturated, so that it spills over into the urine. (It should be noted here that greater bicarbonate reabsorption occurs when the partial pressure of CO2 in the blood is elevated above the normal, and conversely, less bicarbonate reabsorption occurs when the partial pressure of CO2 is lower than normal. The significance of this will be indicated in the next section). The processes of recovery from a metabolic alkalosis are shown in Figure 326.
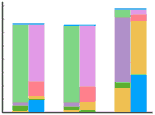 |
Regulation of pH by Kidneys and Lungs:
Figure 327 shows the structure of the extracellular and intracellular fluid. For each set, the vertical bar at the left corresponds to the cations of plasma; the bar at the right, of equal height, corresponds to the anions. The horizontal bar across the top describes the concentration of H2CO3.
One may get a quick picture of the pH of the extracellular fluid by concentrating on the carbonic acid-bicarbonate buffer system; the other ions of the extracellular fluid contribute very little to the pH. Recall that the pH of the buffer mixture depends on the ratio between its basic and acidic parts, not on the absolute value of either.
In Figure 329, the blue bar showing H2CO3 is equivalent to 1.2 mM, the red bar showing HCO3- is 24 mM. The ratio of HCO3- / H2CO3 is 20, so the pH according to the Henderson-Hasselbach equation is 7.4.
The next stage shows the dislocation produced by the production of a metabolic acid. HCO3- is shown to be reduced, while H2CO3 rises. The ratio of HCO3- / H2CO3 therefore falls, so the pH must fall. This describes an uncompensated metabolic acidosis.
The high level of H2CO3 results in increased respiratory activity and H2CO3 falls. This fall, together with a proportionately equal fall in HCO3, results in the restitution of normal pH, and this may be spoken of as a compensated metabolic acidosis. See the final stage of Figure 329.
Decreased respiratory activity results in an increase in H2CO3. If the respiratory system is not responsive to the increased H+ and CO2, an acidosis is produced. Beginning with respiratory inadequacy, this picture may be called respiratory acidosis. The second stage of Figure 331 shows the structure of the body fluids when this condition is uncompensated.
The higher partial pressure of CO2 increases the the kidneys' reabsorption of bicarbonate. This leads to the final changes shown in Figure 331, which represent compensated respiratory acidosis.
Secretion of hydrochloric acid by the stomach results in an increase in HCO3- in the plasma. This is shown in Figure 333, an uncompensated metabolic alkalosis.
The higher pH in this condition leads to reduced respiratory activity. At the same time, the kidneys rejects some of the elevated HCO3-, restoring it toward normal. The result is shown in the final segment of Figure 333, a compensated metabolic alkalosis.
Hyperventilation, whether deliberate or unconscious, lowers H2CO3 and raises the pH. This is an uncompensated respiratory alkalosis. This very often produces a major problem in nervous people who express their sorrows in frequent sighs. Such unconscious hyperventilation over a long period of time may result in pH changes so great that some normal functions become disordered. Figure 335 shows the appearance of the bicarbonate-carbonic acid system in such a person, as well as the partial correction through diminished renal reabsorption of HCO3-, a compensated respiratory alkalosis.
The above conditions illustrate some of the attacks which are made on the body fluid pH and the way in which the kidneys and lungs coordinate their responses to correct these attacks. It should be borne in mind that the compensations are rarely perfect.
6. Regulation of Extracellular Ions:
The concentration of sodium ion
in the extracellular fluid is regulated by the mechanism which controls osmolarity
of the body fluids. As has been noted, the exact mechanism
which regulates the total amount of sodium in the body is not known, but the adrenal
cortex, through aldosterone, may play a significant role in this regulation.
Aldosterone probably acts to increase the reabsorption of sodium from the distal
tubule. At the same time it increases the excretion of potassium, and it is possible
that the two ions are simply exchanged for each other.
The mechanism of potassium regulation
is not fully known. Potassium passes into the glomerular filtrate along with other
diffusable materials, but is apparently reabsorbed completely in the proximal
tubule
The calcium ion is controlled by the parathyroid hormone and the thyroid hormone calcitonin (Chapter 25). The greatest part of calcium regulation has to do with its movement into and out of bone, but renal reabsorption can also vary. An interesting aspect of calcium regulation is seen in alkalosis, whether respiratory or renal. In alkalosis the binding of plasma calcium by proteins is increased; thus less circulates unbound. A low level of unbound calcium leads to muscular twitching and cramps. This is one of the distressing complications of involuntary hyperventilation.
There is virtually nothing known about the renal handling of magnesium. There is some evidence which suggests that magnesium responds to the same factors that influence calcium; when aldosterone is secreted it responds like potassium.
Chloride is normally kept at about 105 mEq/L; there does not seem to be any specific mechanism for its handling by the kidney.
Sulfate and phosphate are both filtered at the glomerulus. Both are slightly reabsorbed in the tubules, but the amount in the urine usually corresponds fairly closely to the product of the glomerular filtration rate and the plasma concentration of these ions. Note: the amounts of these substances which must be excreted correspond to the rate of degradation of sulfur and phosphorus containing compounds. When the glomerular filtration rate is reduced, the amount of these substances eliminated remains the same; but because of the lower filtration rate there is a higher plasma concentration of both.
Bicarbonate in excess of 28 mEq/L, as has been noted, simply exceeds the reabsorptive capacity of the tubules and is eliminated in the urine. When bicarbonate is low, the kidney appears to be able to synthesize it, replacing other anions with bicarbonate. The replaced anions are excreted while bicarbonate is restored to the plasma. This ability to supply bicarbonate to the body is the kidneys main weapon in dealing with metabolic acidosis. In a sense, the kidney corrects the error made with respect to bicarbonate when acid metabolites are produced.
7. The Extracellular Fluid in Renal Failure:
Renal failure, as in late glomerulonephritis, is associated with a wide variety of changes. Low glomerular filtration rates result in rising concentration of sulfate and phosphate. As phosphate rises, calcium falls, provoking muscle contractions and spasms. The lost ability to produce bicarbonate results in progressively worsening acidosis; in time hydrogen ion concentrations may rise so high as to replace a significant part of the intracellular potassium. This potassium, released into the extracellular fluid, cannot be effectively excreted by the damaged kidney. As its concentration rises, the initiation and conduction of the cardiac impulse are impaired. Death usually occurs before extracellular potassium reaches 10 mEq/L.
8. Artificial Kidneys:
The kidneys are remarkably complicated organs in which many obscure chemical processes go on at once. It is therefore quite surprising that they are easily replaced by fairly simple artificial devices in which no chemical transformations occur at all. Basically, all artificial kidneys expose the blood of the patient to an "ideal" extracellular fluid across a cellophane membrane. The membrane is usually quite thin and has a large surface area so that molecules and ions can dialyze across it rapidly. Blood from the patient can be driven by arterial pressure or pumped into the dialyzing tube; after having passed through the tubing it is returned to a vein.
In the first few years after the artificial kidneys were developed, there were numerous problems with them. Many of these have now been solved. For example, Teflon catheters are now implanted permanently into the artery and vein used in the procedure. These catheters cause little tissue reaction and they rarely clot. When they are not connected to the artificial kidney, they are connected to each other making a small arteriovenous shunt. Clotting problems during the period of dialysis are handled by introducing heparin at the arterial end of the dialysis machine and neutralizing it with an antagonist just before it reenters the patient.
The fluid on the other side of the tubing (the ideal extra-cellular fluid) usually contains sodium, potassium, calcium, magnesium and chloride at approximately the right concentrations for extra-cellular fluid and bicarbonate in somewhat higher concentration (36 mEq/L). As the blood passes through the apparatus, it loses such substances as phosphate and sulfate, excess potassium, urea, uric acid and creatinine and returns to the patient with the concentration of the substances chosen for the dialyzing fluid (It is necessary to use very large amounts of dialyzing fluids-from 100 to 300 L).
The cost of using artificial kidneys was, at first, prohibitively high-on the order of $420,000 a year. At present, it seems quite possible that the cost may be reduced to as little as $2,000 a year. It is unlikely to go much below this value, since a certain amount of professional time will inevitably be involved in the management of every dialysis patient. Most patients do best with two dialyses a week each 8 hours long, but dialysis once a week or every other day both give fairly satisfactory results.
Continue to Chapter 24.