Core-Shell Particles
Caution: Since the colloid synthesis is sensitive
to various laboratory conditions and the practices of the individual
researchers, the following procedure should be taken as a general
guideline. One may have to vary the experimental conditions to suit
their needs.
- Gold Capped
Silica/Alumina
Collods
- Gold Capped
TiO2
Collods (TiO2@Au)
- Ag @TiO2 and Ag@SiO2
Colloids.
Gold Capped
Silica/Alumina
Collods
Nalco Chemicals markets alumina capped silica colloids that are stable
in acidic pH. These colloids are positively charged and
electrostatically bind to [AuCl4]-
ions
- Prepare 10mM HAuCl4 solution
(0.0985g in 25mL of H2O).
- Add 20 mL of the 10 mM HAuCl4
solution dropwise with vigorous stirring to 20mL solution of 10% SiO2/Al2O3
solution in water (2mL of SiO2/Al2O3 in 20 mL of H2O).
I used a solution consisting of 3.8 (w/w)% Al2O3 and 19 (w/w)% SiO2.
- Add sodium borohydride solution in water (~10mM) dropwise with
vigorous stirring until a color change from pale yellow to dark purple
occurs.
See reference, Dawson, A; Kamat, P. V. J. Phys. Chem. B.
2000, 104,11842-11846
Gold Capped TiO2
Collods (TiO2@Au)
5mM TiO2 Colloids:
- Prepare 10% titanium(IV) isopropoxide in 1-propanol (or
isopropanol):
Dilute 2.5mL of titanium(IV) isopropoxide in 25mL of 1-propanol.
- Prepare 5mM TiO2:
2.97mL of 10% titanium(IV) isopropoxide diluted in 200mL of water.
pH of water was adjusted to 1.5 using 1M HClO4
prior to addition, to stabilize TiO2 colloids.
**IMPORTANT: Add titanium isopropoxide solution slowly,
1 drop at a time, while stirring vigorously, to obtain a clear,
colorless solution.
- Store in a stoppered flask and use freshly prepared.
Note: I was able to make TiO2
colloids of concentrations of up to 15mM. Higher concentrations were
less stable and the resulting solutions were whitish and cloudy (not
suitable for absorption measurements).
5mM HAuCl4:
- Dissolve 0.1697g of HAuCl4 in
100mL water.
TiO2@Au Colloids:
- Add desired volume of Au dropwise to TiO2
colloids while stirring vigorously, to obtain desired concentration.
- I generally worked with [TiO2] of 2-4mM and
[Au] of 0.06-0.3mM
- Note:
For very low [TiO2] or very high [Au]
(with respect to TiO2) then the colloids are
unstable.
- Stir for 5-10 min (to allow all gold to adsorb to TiO2 surface).
BH4- Reduction:
- Prepare ~10 mM NaBH4 in water
(use 1mM when TiO2 concentration is low to avoid
aggregation effects):
0.0095g NaBH4 dissolved in 25mL
of water.
- Add NaBH4 dropwise to solution
with vigorous stirring until a color change to ‘wine red’ is seen (some
gas will be released).
**IMPORTANT: Initially a color change to dark purple will
be seen. Continue adding NaBH4 beyond
this initial color change until the ‘wine red’ color is achieved. If
the color remains a dark purple despite the addition of more NaBH4 then the colloids have aggregated. To avoid
this, decrease the NaBH4 concentration and add at a slower rate.
It is also important to note that if the TiO2
concentration is too low, or the metal concentration is very high then
aggregation is likely to occur.
- Continue stirring until gas is no longer forming, then top
solution
to desired volume using water with pH adjusted to 1.5 (with HClO4).
Dawson,
A. and Kamat, P. V., Semiconductor-metal nanocomposites.
Photoinduced fusion and photocatalysis of gold-capped TiO2 (TiO2/Au)
nanoparticles. J. Phys. Chem. B, 2001, 105, pp 960-966.
Ag @TiO2 and Ag@SiO2
Colloids.
The synthesis of silver core and TiO2 shell requires
patience and careful optimization of experimental condictions. If not
carefull the TiO2 clusters are formed separately. The method
adopted is as follows
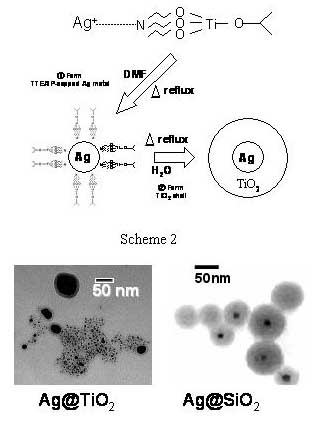 Desired
concentration of TTEAIP (8.3mM, unless otherwise specified) was
prepared in iso-propanol.
Two mL of 15mM AgNO3 solution was mixed with 18mL of TTEAIP
solution. Ten mL of DMF was then added into TTEAIP-Ag solution. The
concentrations of Ag+ and TTEAIP in this solution are 1 mM
and 5mM, respectively.
Desired
concentration of TTEAIP (8.3mM, unless otherwise specified) was
prepared in iso-propanol.
Two mL of 15mM AgNO3 solution was mixed with 18mL of TTEAIP
solution. Ten mL of DMF was then added into TTEAIP-Ag solution. The
concentrations of Ag+ and TTEAIP in this solution are 1 mM
and 5mM, respectively.
The
volume ratio of DMF and i-PrOH has been optimized by carrying out
several batch preparations.
When the amount of DMF was too little or when i-PrOH was excluded,
aggregation
of clusters is observed. The volume ratio of DMF and i-PrOH hence is an
important factor in the preparation of the Ag @TiO2
clusters.
The
solution was stirred first for 15 minutes at room temperature and then
refluxed
with continued stirring. With continued heating of the solution, the
color
slowly changed from colorless to light brown. After 90 min, the color
of the
suspension turned to dark brown. At this point the heating was stopped
and the suspension
was stirred until it cooled down to room
temperature. Thesuspension exhibits a plasmon absorption at 460
nm and shifts to 420 nm upon UV-irradiation in N2 purged solutions.
Silica capped Ag particles
(viz., Ag @SiO2 ) were prepared using active silica
instead
of TTAEIP. The
cluster suspension of Ag@TiO2 and Ag@SiO2 was
centrifuged
and resuspended in ethanol solution. The
procedure was repeated at least 3-times to minimize the content of
water and
DMF in the suspension.
Hirakawa, T. and Kamat, P. V., Electron Storage and Surface
Plasmon Modulation in Ag@TiO2 Clusters. Langmuir, 2004, 20,
5645-5647.
Pastoriza-Santos, I., Koktysh, D. S., Mamedov, A. A.,
Giersig, M., Kotov, N. A. and Liz-Marzan, L. M., One-pot synthesis of
Ag@TiO2 core-shell nanoparticles and their layer-by-layer assembly.
Langmuir, 2000, 16, pp 2731-2735.
Ung, T., Liz-Marzan, L. M. and Mulvaney, P., Controlled
method for silica coating of silver colloids. Influence of coating on
the rate of chemical reactions. Langmuir, 1998, 14, pp 3740-3748.

 Desired
concentration of TTEAIP (8.3mM, unless otherwise specified) was
prepared in iso-propanol.
Two mL of 15mM AgNO3 solution was mixed with 18mL of TTEAIP
solution. Ten mL of DMF was then added into TTEAIP-Ag solution. The
concentrations of Ag+ and TTEAIP in this solution are 1 mM
and 5mM, respectively.
Desired
concentration of TTEAIP (8.3mM, unless otherwise specified) was
prepared in iso-propanol.
Two mL of 15mM AgNO3 solution was mixed with 18mL of TTEAIP
solution. Ten mL of DMF was then added into TTEAIP-Ag solution. The
concentrations of Ag+ and TTEAIP in this solution are 1 mM
and 5mM, respectively.