Unit IV-Circulatory System
Chapter 10
Anatomy and Physiology of the Heart
 |
 |
 |
1. Size and Location of the Heart:
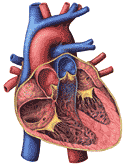
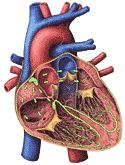
The human heart is about the size and shape of a closed fist. It lies in the chest between the second and fifth ribs, most of it being on the left side of the body. The heart is best seen on plates 3 and 5 of the Dissectograph, where it is numbered 35. The relationship of the heart to the rib cage is shown in Figure 200. The general structures of the heart are shown in Figure 201a.
2. Pericardium:
When the chest is opened and the heart approached, it is seen to be surrounded by a tough sheet of tissue. This is the fibrous pericardium. This is lined by the serous pericardium, a double layered sac which covers the surface of the heart on the outside and the fibrous pericardium on the inside. There is a potential space between these two layers which ordinarily glide freely along each other. This arrangement is shown in Figure 201b. This figure also shows the relationship between the outside lining of the heart (also called the epicardium), the heart muscle (or myocardium), and the inside lining of the heart (endocardium).
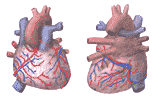
3. Coronary Circulation:
The blood vessels which supply the myocardium form a distinct circulation called the coronary circulation. The coronary arteries and veins lie between the two layers of the serous pericardium, atop the epicardium. Figure 202 shows the coronary circulation as seen from the back and the front of the heart.
4. Myocardium:
The myocardium or heart muscle is best thought of as two separate muscles, each so partitioned as to make two chambers. Blood which enters the heart from the body comes by way of the superior and inferior vena cava and the agygos vein to the first chamber, the right atrium. The next chamber is the right ventricle.
From here blood is ejected to the lungs via the pulmonary artery. Return from the lungs is to the left atrium, from which blood flows to the left ventricle. The left ventricle ejects blood into the aorta from which it is distributed to the entire body. The two atria and the wall between them (interatrial septum) are a single muscle; so too are the two ventricles and the inter ventricular septum. The connection between the atria and the ventricular muscle is by way of specialized conducting tissue--the atrio-ventricular node (See also Part 11). The relationship of the four chambers of the heart is best seen schematically (Figure 203).
5. Endocardium:
The endocardium is more than the lining of the chambers of the heart. Its foldings make the valves which separate atria and ventricles and the ventricles from the pulmonary artery and the aorta. It is shown in Figure 201b. The myocardium in this figure is continuously lined by endocardium. Sheets of endocardium separate the atria and the ventricles and small endocardial flaps separate the ventricles from their respective outflow arteries.
6. Chambers and Valves of the Right Heart:
The blood from the body enters the right atrium and passes through the three cusped right atrio-ventricular valve into the right ventricle. The atrioventricular valve edges are "anchored" by tendinous cords to the papillary muscles which are a part of the right ventricular myocardium and contract with it. The blood is ejected from the right ventricle into the pulmonary artery past the pulmonary arterial valve. See Figure 201a.
7. Chambers and Valves of the Left Heart:
The main points of difference between the left and right sides of the heart have to do with the atrio-ventricular valve (two cusped on the left) and the greater thickness of the ventricular wall on the left. The two cusped atrio-ventricular valve is called the mitral valve. The valve in the aorta, three cusped like that in the pulmonary artery, is called the aortic valve. See Figure 201a.
8. Pattern of Blood Flow Through the Chambers of the Heart:
Injection of radio-opaque material into the right heart, followed by X-ray cinematography makes possible the visualization of the normal course of blood flow and gives a sense of the timing. Diagrams of such X-ray motion pictures are shown in Figure 207.
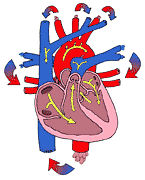
9. Conduction System: The Sinoatrial Node:
 |
The sinoatrial node is a comma shaped group of cells lying in the wall of the right atrium just where it is entered by the superior vena cava. These cells have a peculiar property: they depolarize intermittently without any external stimulus. Repolarization begins immediately, but is rather slow. Even during the repolarization process these cells begin the permeability changes which result in the next depolarization. In dogs this process appears to occur about 70 times per minute.
The depolarization and
repolarization at the sinoatrial node
serve as a stimulus for the adjoining muscle of the atria. This tissue is
stimulated, and is depolarized, stimulating the muscle adjoining. Eventually the
entire atrial musculature is involved. The impulse which initiated the atrial
depolarization spreads from the sinoatrial node over the atrial tissue at about 1
meter per second, and the sinoatrial node from which the impulse normally
originates is called the pacemaker of the heart.
10. Conduction System: Electrical Separation Between Atria and
Ventricles:
The depolarization wave which
sweeps across the atria does not
enter the ventricle directly, for the atria and ventricles are separated from
each other by non-excitable tissue--the atrio ventricular ring. Events which
occur in the atria are detected by the ventricles only when they enter the
ventricular system by way of the atrio ventricular node.
11. Conduction System: Atrio Ventricular Node:
The atrio ventricular node
sits atop the
interventricular septum, as a rider on a saddle. Its upper end lies in the
interatrial septum, its lower end is replaced by specialized conducting tissue.
Depolarization of the adjacent atrial tissue is the stimulus for depolarization
of the atrioventricular node. The impulse passes through this node by a
propagated depolarization much like that seen in the atria, but its velocity is
much slower, about 10 cm/sec.
12. Conduction System of Ventricles:
Once the atrio ventricular
node has been stimulated and
the impulse has passed through it, it is propagated very rapidly through the
entire ventricular system, first by specialized conduction tissue, (the
bundle of His and the bundle branches) then by ramifications of
these branches called Purkinge tissue, just under the endocardial surface
of the heart, and finally by the ventricular muscle itself from the
endocardial to the epicardial surface. The speed of this conduction
is very great; from the moment the impulse enters the bundle of his to total
depolarization of the ventricles is usually about 0.1 seconds or less. The entire
conduction system of the heart is shown in
Figure 201c.
13. The Normal E1ectrocardiogram:
The events associated with
conduction of the cardiac impulse can
be measured electrically at the surface of the body. The record of the electrical
changes is called the electrocardiogram. A typical electrocardiogram is
shown in Figure 208 below. It is easiest to understand the electrical changes by
referring to the inserts which show the condition of the impulse with respect to
the electrocardiographic wave.
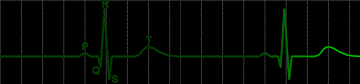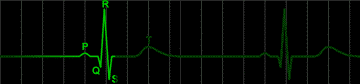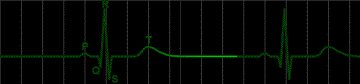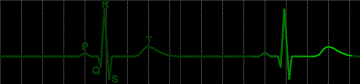 |
fig 208. Normal ECG (Refresh this animation) |
The resting heart, completely polarized, generates no potential, and the electrocardiogram is said at this time to be iso-electric.
The beginning of depolarization of the pacemaker generates a small potential between the depolarized area and the normal area. The potential is small because the electrical couple which generates the potential has one small member, i. e., the small area of depolarization.
As more of the atrial tissue becomes depolarized, the potential increases in size, until about half of the atrial tissue is depolarized. Now the potential decreases, for the electrical couple again has a small member; this time it is the small area which remains polarized. When the entire atrial mass is depolarized, the potential actually disappears and iso-electricity is restored.
The depolarized atrium does not form a couple with the ventricle since, as pointed out above (Part 10) there is electrical separation between atria and ventricles. A very small couple results from partial depolarization of the A-V node, but it is not detectable in the electrocardiogram.
Once the impulse has passed through the A-V node it depolarizes very large masses of ventricular tissue very rapidly. The couples developed are large and quite transient. They are large because of the large mass of tissue involved and transient because of the speed of total depolarization.
The electrocardiographic wave associated with the process of ventricular depolarization is called the QRS complex. Iso-electricity occurs when the ventricles are completely depolarized, since there is no polarized area to make a limb of the electrical couple.
Repolarization of the ventricular muscle follows an odd course. It would be expected that repolarization would occur first in the areas first depolarized. This would make electrical couples inverted from the normal direction. In fact, the last portion depolarized is the first portion repolarized, so that the electrical couple is in the same direction whether the ventricle is being depolarized or repolarized. The difference lies in the speed of repolarization compared to the speed of depolarization. The repolarization is much slower. Thus an. electrocardiographic wave is generated which is, in general, in the same direction as the QRS complex, but longer in duration. When repolarization is complete, the electrocardiagram becomes iso-electric again.
14. Systole and Diastole Defined:
The electrical events described above merely herald the mechanical events of the cardiac cycle. These events will be described in the next two sections. Contraction of any chamber of the heart is called systole; relaxation is called diastole. The chamber in question should always be specified. Thus atrial diastole and ventricular systole may occur together.
When the term systole is used without qualification it usually refers to ventricular systole; and in the same way, diastole without qualification may be taken to mean ventricular diastole. This type of usage, though common, is very confusing. For example atrial systole normally occurring before ventricular systole is said to give rise to a pre-systolic pressure change, noise or murmur; yet the atrium is itself in systole. In this text, systole and diastole will always be qualified.
15. Internal Configuration of the Heart in the Cardiac Cycle:
When atrial systole begins, the diastolic ventricular tissue is pulled up around the blood formerly in the atria. Likewise, when ventricular systole begins to move blood into the aorta and pulmonary artery, the atrial wall is stretched; the blood which was in the ventricular chambers comes to be in the large arteries by a downward movement of the aortic and pulmonary valves. The effect is that the beating heart hardly seems to change its external configuration with the heart beat. The internal configuration is, however, radically altered (See Figure 210 and 211).
 |
16. Cardiac Cycle:
Description of the cardiac cycle requires that the following be co-ordinated.
A detailed description is given below. Before presenting this description, however, a quick resume will be given.
The cardiac impulse, originating in the S-A node causes atrial systole and, after a short time, ventricular systole. Ventricular systole closes the atrio ventricular valves and opens the valves of the aorta and pulmonary artery. The closure of the atrio ventricular valves gives rise to the first heart sound. Ventricular diastole follows ventricular systole and the aortic valves close. This closure gives rise to the second heart sound.
The cardiac cycle is illustrated in Figure 209. For clarity, the events on the left side of the heart only are shown.
The lowest graph shows heart sounds, and the next lowest tracing is the electrocardiogram. Also note that the time scale has been enlarged to permit the description of many events in a short period of time.
The green tracing in the top graph describes the atrial pressure. Some aspects of this may be difficult to understand until the whole cycle is put together. During diastole of both atrium and ventricles, the pressure is basically steady (isobaric). When atrial systole begins, there is a moderate rise in atrial pressure, blood moving away from the atrium into the ventricle and the pulmonary veins. As the blood leaves the atrium the pressure falls, becoming again isobaric. Atrial diastole and ventricular systole now occur. The expected pressure fall of atrial diastole is not seen; the ventricular systole, which closes the atrio ventricular valve, causes the valve cusps to bulge into the atrial cavity and atrial pressure actually rises. The rest of the atrial pressure tracing depends on ventricular activity. As the ventricle ejects blood into the aorta, the atrio ventricular ring is pulled downward. This enlarges the atrial cavity and lowers its pressure. Blood "sucked" into the low pressure area raises atrial pressure. The ventricle now relaxes and the atrio ventricular ring is moved upward, the valve being open. The pressure in the atria falls as the valve opens and the ventricle begins its diastole.
The pressure changes in the ventricle dominate the whole cardiac cycle and are represented as the yellow line in Figure 209.
Initially, the ventricle is shown as isobaric. Atrial systole directing some blood into the ventricle raises its pressure a little, but it usually falls to the isobaric level. Most of the fall is due to the fact that the ventricular muscle in diastole is easily stretched without pressure change. The arrival of the cardiac impulse at the ventricle causes ventricular contraction. The first effect of the contraction is to close the atrio ventricular valve. The contraction of the ventricle now continues against two closed valves. Pressure rises steeply, but no blood is moved. This is called isometric contraction.
Isometric contraction is terminated when ventricular pressure exceeds the pressure in the aorta. The aortic valve opens and the ventricle moves its contents past the valve at first rapidly, then slowly, and finally not at all. The first two phases are called rapid ejection and diminishing ejection. The third phase marks the moment when the energy for ventricular contraction has all been used. The ventricle now begins its diastole.
The first event of ventricular diastole is closure of the aortic valve. The ventricle, now relaxing between two closed valves shows a precipitous decline in blood pressure. This is called isometric relaxation, and is terminated at the moment that intra ventricular pressure falls below atrial pressure and the atrioventricular valve opens. The opening of the valve and the upward movement of the atrioventricular ring results in a transfer of blood from the atrium into the ventricle. This is called the phase of rapid filling.
It should be noted that rapid filling is a process in which the heart is reshaped so that the blood lies within the ventricular cavity. It is not so much that the blood itself moves as that the ventricle moves up around it.
The aortic pressure curve is the highest tracing. Beginning at the start of the cycle, aortic pressure fades away as the blood leaves for the aortic branches and the peripheral circulation. The monotonic fall is interrupted by ventricular activity, opening the aortic valve. The pressure in the aorta at this time is at its lowest value during the cycle.
The aorta and ventricle are now one chamber and the pressure changes of both vary together during rapid ejection and diminishing ejection. When ventricular diastole begins, the aortic valve snaps shut. The overdistended aorta lets its blood fall against the shut valve, and as is to be expected, it bounces, producing a small secondary pressure rise. During the rest of the cycle, this pressure fades away.
The position of the aortic valve is shown by the lower bar through the top of Figure 209. When the bar is grey, the valve is shut. When it is red, the valve is open. The position of the atrioventricular valve is shown by the uppermost bar--the same conventions are used. Note that there are two times in the cardiac cycle when the valves are both shut. These correspond precisely to isometric contraction and relaxation.
Ordinarily, the heart sounds detectable by the human ear are associated with valve closure. The first sound in the cycle is shown to occur at the moment of closure of the atrioventricular valve. It is a low pitched, fairly long lasting sound, best imitated by the syllable "lub". The second sound, associated with closure of the aortic valve is usually high pitched and quite short in duration and is imitated by the syllable "dup". Murmurs and other sounds will be discussed in Part 20 of this chapter.
17. The Power of the Heart:
The power of the heart is best considered by taking the right and left sides of the heart separately. The right side of the heart must take the blood which is returned to it from the body and expel it through the blood vessels to the lungs. The left side of the heart must accept the blood which is returned to it from the lungs and expel it into the aorta and its branches. The arrangement is shown in Figure 203.
The work of the right heart involves raising a quantity of blood to the pressure which exists in the pulmonary artery. It is easiest to understand this and the following by considering the work involved in using a hand pump to force fluid through a narrow tube. At a given rate of pumping the pressure in the tube depends on how narrow it is. It takes much more pressure and therefore more work to force the same volume through a narrow tube than a wider one. In addition, more work must be done when the volume to be handled is greater against a constant pressure.
In the circulation, the heart corresponds to the pump and the tube corresponds to the arterioles which are the smallest branches of the arteries. The right heart operates against what corresponds to a wide opening; the left heart operates against a much smaller opening. Correspondingly the pressure against which the right heart must operate is much smaller (1/5) than the pressure against which the left heart must operate. Consequently, the right heart does much less work than the left.
It can be calculated that the work of the right heart per minute is the same as that done by a 1/4 watt engine. The work of the left heart is approximately that of a 1 1/4 watt engine. The two sides of the heart working together thus represent only a 11/2 watt engine. This is the same as a 1/500 horse power motor. An ordinary light bulb uses 60 times as much power as the working heart.
The most remarkable feature of the heart as an engine is its ability to adapt to the changes in the work required of it. For example, during exercise the work of the right heart may increase ten times; that of the left heart may increase as much as five times. The heart is able to adapt itself to the increased work requirement and to return to normal conditions following exercise.
18. Measurement of Cardiac Output:
The volume of blood ejected is the same on both sides of the heart. If it were not so, blood would accumulate in the veins behind one or the other sides of the heart. If the left heart ejected more than the right, blood would pool in the veins of the body. If the right heart ejected more than the left, the blood would accumulate in the veins of the lungs. This volume which must be handled by both sides of the heart is spoken of as the cardiac output. It is normally about 6 liters per minute. It may be measured in man by a number of methods most of which involve the determination of the composition of the blood as it changes between the pulmonary artery and the aorta. Since oxygen is added to the blood at the level of the lungs, it is obvious that the concentration of oxygen will be greater in the aortic blood than it is in the pulmonary arterial blood. If one knows this concentration difference, and the amount of oxygen which is taken up in the lungs is measured, the output of the heart can be calculated.
In order to obtain blood samples from the pulmonary artery it is usually necessary to introduce a long flexible tube by way of a vein, using a fluoroscope to guide it and to advance its end through the right atrium, the right ventricle, past the pulmonary arterial valves, and into the pulmonary artery. Blood samples can now be taken from the end of this tube. This procedure, called cardiac catheterization, is quite safe, but is normally carried out only in hospitals. It should be noted here that cardiac catheterization, together with x-ray techniques, makes possible the diagnosis of defects within the heart as well as the sampling of blood from the pulmonary artery.
The measurement of oxygen uptake by the lungs is easily accomplished by measuring the disappearance of oxygen from a closed system from which the patient breathes. A sample calculation of cardiac output follows: Assume that the patient has consumed oxygen at the rate of 300 ml per minute and that the arterial oxygen concentration is found to be 190 ml per liter and that the venous oxygen concentration measured in the pulmonary artery is found to be 140 ml per liter. It is obvious from this that each liter of blood picked up 50 ml of oxygen on its way through the lungs. Since 300 ml of oxygen were taken up by the lungs, 6 liters (300/50) of blood must have passed through the lungs in the minute under consideration. In general this calculation can be described by the following equation:
Cardiac Output = O2 uptake / (Arterial O2 concentration-Pulmonary Arterial O2 concentration),
where the O2 uptake is measured in ml per minute and the arterial concentrations in ml per liter. This is called the Fick equation.
The cardiac output can also be determined by injecting a dye into any vein and determining its concentration in any branch of the aorta. When the cardiac output is large, the dye concentration in the artery is small; conversely, a small cardiac output results in a high dye concentration. This procedure for the measurement of cardiac output has the advantage that it does not require a cardiac catheterization. The method is called the Stewart-Hamilton method; the student who is interested may find the details in textbooks of medical physiology.
The work of the heart depends not only on the amount of blood which it handles but also on the resistance against which it is working. Referring to the analogy used earlier in this chapter, it is more difficult to pump fluid through a tube whose opening is narrow than one with a wide opening. The pressure in the first case is high, the pressure in the second case is low.
In the case of the circulation, the pumping power is provided by the myocardium; the tube in the case of the right heart is the pulmonary artery and the opening corresponds to the size of lung arterioles. In the case of the left heart, the tube is the aorta and the opening corresponds to the condition of the arterioles of all the organs. The resistance which these present to the flow of blood is sometimes referred to as the total peripheral resistance.
The resistance of the pulmonary circulation is much lower than that of the systemic circulation and it is for this reason that the pulmonary arterial pressure is much lower than aortic pressure. Another consequence is that the right heart, which does much less work than the left, has much less muscle than the left.
The cardiac output, which is normally 5 to 6 liters per minute, may increase to about four times that value. The total peripheral resistance may increase to twice normal value in the disease called arterial hypertension (high blood pressure). High pressure may also occur in the pulmonary artery when there is an increase in the resistance of the arterioles of the lung. This will be considered in more detail later.
19.Adaptability of Heart Power:
We must now ask by what means the heart, normally a 1 1/2 watt engine, can adjust itself to an increase in the demands of the body when it requires a six watt engine. Any one of three methods can be employed by the heart to increase its power.
The first is to increase its rate. If the work that the heart does per beat is the same, an increase in rate will result in an increase in power (work/minute). This, however, is not quite enough. For example, a resting man may have a heart rate of 70; during violent exercise, when the power requirement is increased 4 times, he may, at the very most , increase his heart rate 2 1/2 times.
In addition to increasing rate, the heart also has the ability to increase its ability to perform work in any one beat. This is done primarily by increasing the strength of muscle contraction. Adrenaline and noradrenaline (which appear in the blood and at sympathetic nerve endings during exercise) bring about this increase in the strength of the beat.
In addition to increasing rate and strength per beat through the presence of favorable chemical substances the heart has a very remarkable property. The more filled it is with blood before its systole, the stronger the beat. The reason for this has not yet been explained, but the consequences are important. If a heart fails, in any one beat, to expel the blood which came to it during the diastole, the blood which remains behind in the ventricle is added to the blood which goes in during the next diastole. This results in a larger end-diastolic volume. The dilated heart now becomes more forceful in its beat even in the absence of any known chemical alterations. This process by which the heart fails, enlarges, and becomes stronger is an illustration of what is called Starling's Law of the Heart. It is especially well seen in persons in whom the heart is not quite able to meet the needs of the circulation by the first two mechanisms.
The manner in which a heart will respond to an increased work load depends very much on the training and condition of the person and the condition of the heart. In general, normal people who are not in peak physical condition respond first by increasing rate, second by increasing the force of contraction, and only last of all by dilation of the chambers. The trained athlete usually responds first by increasing the force of contraction and second by increasing the heart rate.
The increase in heart rate which occurs after exercise can, in fact, be used to assess the condition of the heart of a person. An unusually great increase in heart rate after exercise probably means that the heart is not up to normal standards. A person in whom the heart rate increases very little after exercise is usually in very good physical condition.
Both the trained and the untrained person do not dilate their hearts until they have used up both of the above mechanisms. When the heart cannot do its work without dilating it may be assumed that there is some degree of impairment of the function of the ventricle; and usually persons with dilated hearts are in the early stages of heart failure. An enlarged heart is usually considered to indicate the existence of heart disease. At first sight, it would appear that if Starling's Law is followed the heart can never fail, for when it begins to fail it becomes enlarged with the blood that was not ejected and therefore becomes more powerful. However, there is an upper limit of dilation past which further dilation of the heart causes a decrease rather than an increase in the force of the heart beat.
A heart which has begun to fail may increase its volume and improve its performance up to this point but if the demands of the circulation are still greater than what the heart is able to accomplish at its new enlarged volume, it will go into complete failure. This is normally prevented by the existence of the pericardium which prevents the heart from overdilating.
As the heart dilates, the pressure within the ventricle increases a little so that there is an increase in the pressure of the veins which empty into that ventricle. If the veins involved are those of the lungs, the increased pressure in the veins results in their engorgement and this leads to difficulty in breathing. When the involved veins are those which empty into the right heart, the veins become distended throughout the body. Anyone who has even seen the swollen neck veins of persons who have been carrying out violent exercise or who have become short of breath as the result of exercise will have, in the first case, been observing the inability of the right heart to cope with the demands of the circulation, and in the second, the inability of the left heart to handle the blood coming to it from the lungs. In a later chapter, we shall see how the next step in this sequence is associated with the formation of excess fluid. In the case of the right heart, this fluid (edema) accumulates through the body. In the case of the left heart the fluid accumulates in the lung and further damages the mechanism involved in the uptake of oxygen. The consequences of right and left heart failure will be discussed in Chapter 16.
Both rate and force of the heart are normally under the control of the autonomic nervous system as well as circulating adrenaline and noradrenaline. The increase in rate is in part the result of the release of catecholamines at nerve endings. The catecholamines also increase the force of the contraction. The mechanism by which they do so is a wasteful one. The utilization of energy by the heart is very considerably increased by adrenaline. At the same time, however, the efficiency with which this energy is turned into useful work is reduced. Normally the increase in energy utilization is greater than the decrease in efficiency, so that adrenaline improves the performance of the heart. In patients with heart disease, however, particularly in those who have disease of the coronary arteries, the ability to deliver the source of energy is impaired and in such people the only effect of adrenaline is to produce reduction in efficiency. Adrenaline should never be used in the treatment of chronic heart failure associated with disease of the coronary arteries.
Overall, using all of these
factors together, the human heart is
probably capable of developing 5 or 6 watts of power.
20. Control of the Heart Rate:
The adaptation of the heart's
output to the requirements of the
body is primarily one of adjusting the heart rate to the cardiac output. This is
usually accomplished by a combination of physical, chemical and nervous factors
acting on the pacemaker, or sinoatrial node.
Physical factors: When the
sinoatrial node is warmed by
blood returning to the heart from an active area, its discharge rate is
increased. The same is true in fevers; the rapid pulse of most fevers is a
familiar illustration. Conversely, cooling the sinoatrial node reduces the rate
of discharge, a response often used in surgery of the heart. Deliberately
produced hypothermia may reduce the heart rate to 10 - 15 beat/min. The
patient is placed in a cold environment, and drugs are given to interfere with
normal temperature regulation. As the body temperature falls, the heart rate
falls, and operations can be performed which cannot be performed on the rapidly
beating heart.
Chemical factors: The
discharge rate of the sinoatrial
node is, to a certain extent, under the control of circulating catecholamines,
which may have been formed elsewhere, either by the adrenal medulla or at other
sympathetic terminals. Acetyl choline, formed elsewhere than the vagal fibers to
the heart, is usually destroyed by cholinesterase before it can reach the
pacemaker. It is an odd fact though that the first successful attempt to show
chemical transmission of the nerve impulse was made by vagal stimulation of the
frog heart. The fluid which passed through the heart during vagus stimulation was
found to be able to slow a second heart. It was named vagusstoffe and
subsequently proved to be acetyl choline: See Figure 218.
Nervous factors: The most
important factor in the control
of heart rate is the activity of the autonomic nervous system on the sinoatrial
node. The accelerator nerves of the sympathetic and the cardiac fibers of the
right vagus appear to act together in this regard. Increased sympathetic
activity, decreased parasympathetic activity, or both may increase heart rate.
Conversely, the heart rate may be reduced by decreased sympathetic activity or
increased parasympathetic activity or both. This is like driving a car with one
foot on the accelerator and one on the brake. Increased speed may result from
increased use of the accelerator and decreased use of the brake or both.
Overall, the autonomic nervous
system translates the body's
needs for cardiac output as they are sensed peripherally and integrated by the
central nervous system. The responses of people with respect to this are quite
variable. For example, some people react to a painful stimulus by increasing
heart rate, increasing sympathetic and decreasing parasympathetic activity.
Others respond with a decrease in heart rate. Strong emotion likewise can produce
either effect. Fainting due to strong emotion may result from heart rate
depression; more usually strong emotion increases the heart rate.
One of the more regular responses
is that to a fall in pressure
of the arterial blood; the sensory stimulus here is decreased stretch in the arch
of the aorta or in the carotid sinus, part of the artery which supplies the brain
(internal carotid artery). The information conveyed to the central nervous system
from these detectors is usually such as to cause increased sympathetic and
decreased parasympathetic activity - the heart rate consequently increases.
Recent results, however, suggest that this may not be nearly so regular a finding
as one would think. The whole mechanism (sensor, pathway to the central nervous
system, center in the central nervous system, vagus and sympathetic nerves) is
called the barostatic reflex.
The reverse is also seen: sudden
rises in arterial pressure
cause decrease in sympathetic and increase in parasympathetic activity; this
usually results in a lowering of heart rate. The change must be a sudden one;
people with chronically high arterial pressures in general have normal heart
rates. The barostatic reflex is also concerned in the regulation of the blood
pressure, and will be discussed further in Chapter 11.
A reflex of less certain
properties is called the Bainbridge
reflex. Increased return of blood to the heart is supposed to be detected by
pressure receptors in the right atrium; the response is mediated via the central
nervous system; cardiac acceleration is supposed to result.
There has been much controversy
about this reflex since it was first described in 1915. It
is not clear what the receptor is; nor what carries the information to the central
nervous system. It is, however, clear that the heart rate increases after a
rapid intravenous infusion provided it was low to begin with.
A practical thing to remember
about the heart rate is concerned
with these reflexes. The heart rate sometimes becomes exceptionally rapid (more
than 160/min) due to abnormal discharges originating in or near the sinoatrial
node. Such rapid hearts spend very little time in diastole, and their filling is
ineffective. The application of pressure at the angle of the jaw (over the
carotid sinus) may excite the sinus nerve and activate the barostatic reflex,
just as if the arterial pressure had risen. This maneuver may be life saving.
21. Disorders of the Heart:
Any of the valves of the heart may
be diseased in such a way
that it does not open completely, usually as the result of adherence of the valve
cusps to each other. This condition is called stenosis.
Insufficiency, on the other hand develops when valve cusps do not close
completely. This usually occurs from dilatation of the ring which seats the
valve.
The rapid advances in cardiac
surgery have made it possible to
repair and replace defective cardiac valves. Methods for diagnosing the sites of
valve damage and the nature of the damage have correspondingly taken on greater
importance.
Usually, a valvular defect is
first indicated by a heart
murmur. The murmurs are ordinarily produced by blood passing in jets
through narrow openings. For example, a stenotic aortic valve will "jet" blood
from the ventricle into the aorta during systole and a systolic murmur will be
heard. An atrioventricular valvular insufficiency will also produce a jet of
blood going from ventricle to atrium in systole. Diastolic murmurs result from
stenosis of the atrioventricular valves or insufficiency of the aortic or
pulmonary arterial valves.
Murmurs can occur in the absence
of disease, particularly over
the aorta during systole when the cardiac output is high. Such functional
murmurs must be distinguished carefully from the murmur of aortic stenosis.
The diagnosis of valvular disease
is also helped by observations
of the pressures controlled by the valves. For example, in aortic insufficiency
the valve, which should close and hold a high level of pressure in the aorta,
lets blood flow back into the left ventricle during its diastole. As a result,
the aortic pressure becomes very low during ventricular diastole. In stenosis of
the valve between the left atrium and left ventricle blood does not flow easily
into the left ventricle. It is, therefore, dammed up behind the valve, producing
a rise in pressure in the veins from the lung. Though this cannot be measured
directly, it can be detected by the fact that the lung becomes swollen with
blood, which produces difficulties in breathing.
Interest in characterizing the
murmurs has resulted in the
development of various phonocardiographs. These devices disclose not only
murmurs, but also extra sounds which are not well heard by the stethescope and
ear. One of these sounds occurs in atrial systole; another is heard just after
isometric relaxation of the ventricle. These sounds, called respectively the "a"
and the third heart sound, are related to the normal heart sounds in the
following sequence:
When either the "a" or
the third heart sound is heard by the ear, a peculiar rhythm called a "gallop" is
detected. Such gallop rhythms are usually indicative of severe damage to the
ventricular wall so great that it is thrown into vibration during the changes in
internal configuration of the heart that occur with atrial systole or ventricular
diastole.
A variety of artificial valves is
now available to replace
damaged ones. These valves are usually made of inert plastics, but present
problems with respect to clotting and hemolysis throughout life. It seems
possible that valve grafts from dead persons (or even animals) may take their
place.
Inflammations of the pericardium
may occur as the result of a
variety of conditions. Most of them are quite benign. Occasionally, however,
fluid accumulate between the layers of the serous pericardium. The chambers of
the heart are prevented from filling normally, sometimes so much that normal
cardiac output cannot be maintained. This calls for draining the fluids, and can
be accomplished by a needle tap on the pericardial sac.
This is perhaps the commonest of
the major illnesses of the
heart. The ability of the heart to perform work is dependent on an adequate
coronary blood flow. When coronary arteries are obstructed, the results range all
the way from chest pain on exercise to sudden death. This will be considered
again in Chapter 16.
a. Valvular Stenosis and Insufficiency:
a First Second Third
b. Pericarditis:
c. Coronary Insufficiency:
 |
fig 220. Atrial Flutter |
Many disorders of the heart beat can be detected in the electrocardiogram. For example, the impulse which begins at the sinoatrial node may begin to pursue a circular course around the vena cava. Everytime the impulse goes around the circle, it will produce a discharge which depolarizes the whole atrium. This condition, called atrial flutter, is shown in Figure 220. The characteristic feature of the electrocardiogram in flutter is the existence of trains of P waves. The ventricle is not usually stimulated by the atrial depolarization of each P wave, but may respond to every second depolarization. This would be called a 2:1 block. An electrocardiogram of this condition is shown in Figure 221.
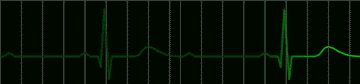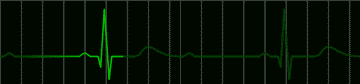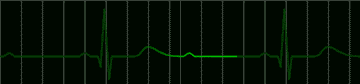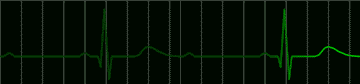 |
fig 221. ECG showing a 2:1 Block (Refresh this animation) |
Disease of the atrial wall may result in a highly irregular "wandering" of the impulse over the atrium. The atria do not contract effectively, but only squirm. The cardiac impulse is transmitted irregularly to the A-V node, and a totally irregular pulse results. This condition is called atrial fibrillation; a colorful name applied to the pulse in such persons is "delarium cordis" or madness of the heart. An electrocardiogram of this condition is shown in Figure 222.
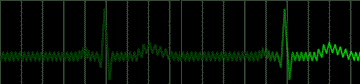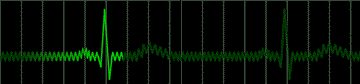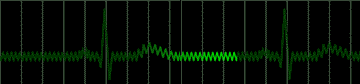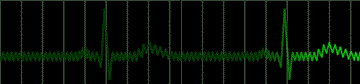 |
fig 222. ECG showing Atrial Fibrillation (Refresh this animation) |
There is an interesting condition in which the atrio ventricular node sometimes follows the pacemaker, but sometimes becomes independent of it. When the A-V node is independent, it sets up its own, slower rhythm. Patients do well with either pacemaker rhythm or A-V nodal rhythm, but during the transition from pacemaker to A-V nodal rhythm they may lose consciousness. This can be remedied by the use of artificial pacemakers which drive the ventricles. Such pacemakers are battery powered. They are easily implanted and they may last for years. A typical electrocardiogram in an untreated patient with this condition is shown in Figure 223.
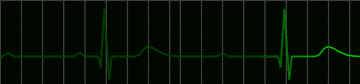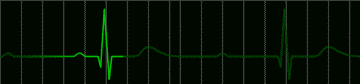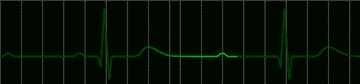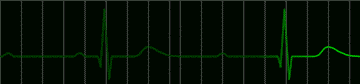 |
fig 223. ECG showing a Complete A-V Block (Refresh this animation) |
Atrial fibrillation makes the contractions of the atria ineffective and those of the ventricles irregular, but it is not life threatening. Ventricular fibrillation was once a terminal event. Now, if recognized immediately, it can be reversed. A defibrillator, which makes use of a direct current to hyperpolarize the heart stops the fibrillation. Within a minute or two the heart is paced from the outside, and may return to normal rhythm. An electrocardiogram during ventricular fibrillation is completely irregular.
The heart, as has been seen, is a very small engine, even when it is working hardest. It should be possible to replace it entirely with two pumps, one of which takes the place of the right heart, delivering blood to the lungs, and one of which takes the place of the left heart, delivering blood to the aorta and its branches. The major problem is not how to power such pumps-this is very easily done-but rather the coupling of the two pumps to each other. For example, a left pump more powerful that the right would result in systemic edema; conversely a right pump too powerful for the left would result in pulmonary edema. The development of a satisfactory artificial heart might easily save a half million lives in the United States alone each year.
Heart assist devices have been used over short periods when the heart is so severely damaged that it cannot maintain enough cardiac output to sustain life. In general, they withdraw blood from the arteries when the ventricle is in systole (so that it works against very little pressure) and reinject the withdrawn blood during diastole. Presumably the pump reduces the power required by the ventricle. Exact timing is necessary; the electrocardiogram is used to signal pump withdrawal and reinfusion. The results are very poor, perhaps because these assist devices are rarely used except in dying patients.
The requirements that a suitable
donor and a suitable recipient
be available at the same time limits the application of this operation.
Troublesome questions have been raised concerning the meaning of death for the
heart donor and the certainty of a bad outlook for the recipient.
All in all, less than 25 patients had heart transplants
within eight months after the first operation and of these only 5 are alive at
the time of this writing.
Nevertheless, it is possible that
the operation will be useful in a limited number of persons
Continue to Chapter 11.