Unit VII - Excretion
Chapter 22
Formation and Excretion of Urine
1. General Remarks:
In the final metabolism of most foods, carbon dioxide and water are formed. CO2 and some of the water are eliminated by the lungs. Other substances taken in or produced in food breakdown are eliminated by the other excretory organs, the skin, large intestine, and the kidneys. Of these the kidneys are the most important. The structure of the kidneys is most easily understood in terms of their function. This will be described first.
The kidneys are charged with regulation of the blood and through it the other fluids of the body (Chapter 23). In order to fulfill this function adequately, the kidneys must "see" large volumes of blood. In fact, the kidneys are presented with almost one quarter of the cardiac output; of the major organs of the body, they receive the largest amount of blood per unit weight per minute. For example, the brain receives half its weight in blood per minute, resting skeletal muscle one tenth, and skin one twentieth. Compared to this, the kidney receives four to five times its weight in blood per minute. These organs, weighing about 300 grams together, are supplied with 1200-1500 ml of blood per minute.
Little of this blood is used for nutritive purposes. In most organs somewhere between one fifth and three fifths of the oxygen is extracted from the arterial blood. In the kidney, the value is about one tenth. Thus, the huge blood supply of the kidney does not occur in response to a metabolic need on the part of the organ; it is, rather, related to the fact that the kidney can only adjust the blood properly if it is brought into contact with a great deal of it.
The manner in which the kidney handles the large volume of blood presented to it involves three processes. It begins with a process called filtration, in which approximately one fifth of the blood plasma coming to the kidney is removed from the blood. (This statement is an approximate one and will be modified later). The discarded materials contain substances which are useful to the organism as well as wastes; those which are useful are to a large extent "recaptured" by the blood in a process called reabsorption. It will be recalled that about one-fifth of the plasma was discarded; about four fifths, however, remains, and some materials destined for excretion are in this fraction. Many of these are added to the discarded part of the plasma by a process called "secretory excretion." Finally, a few substances are actually created by the kidney from others in the plasma and added to the urine. This process, a very important one, is not usually named; it may be called "synthesis".
Urine, then, represents discarded plasma, from which certain substances are reclaimed, and to which other substances normally present in plasma are added; finally the kidney makes certain substances for the urine from other substances in the plasma.
2. The Nephron:
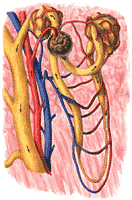
Each kidney in man contains about one million more or less similar units which carry out the above functions. These are called the nephrons. Nephrons are made of parts derived from the vascular system and parts derived from the kidney itself. The latter are sometimes referred to as parenchymatous.
 |
The structure of a nephron is shown in Figure 308. The formation of the urine begins in the capillaries at the left. These capillaries are unlike other capillaries in that their blood is at very high pressure; this is a consequence of the fact that they have a short, wide arteriole, called the afferent arteriole. Two other differences from other capillaries should be stressed: These capillaries form little clusters, which in the low power microscope look like mulberries (glomeruli=little mulberries), and they are followed not by a venule, as one might expect, but rather by another arteriole, called the efferent arteriole. It seems probable that the efferent arteriole, through its contraction, can add to the already high pressure in the glomerular capillaries.
Despite these differences from other capillaries, the glomerular capillaries obey the same laws as other capillaries. They extravasate fluids when capillary pressure is higher than the sum of colloid osmotic pressure and tissue pressure and at a rate which is directly proportional to this difference. However, because their pressures are so high, they rarely intravasate fluids. Consequently, the glomerular capillaries almost always leak.
The fluid extravasated, since it can not return to the glomerular capillary goes into the tissue space of the kidney. Here, the parenchymatous portion of the kidney is beautifully modified to act as a collecting cup for the extravasated fluid. This cup is called Bowman's capsule. It can be likened to a funnel with a filter paper: its inner layer, intimately related to the glomerular capillaries, is like the filter paper; its outer layer is like the wall of the funnel. The fluid collected in Bowman's capsule is an ultrafiltrate of plasma. It seems to be quite protein free; otherwise it is just like the plasma in composition. It is formed at a rather impressive rate. Though only about one fifth of the plasma is filtered into the capsule, so much blood comes to the capsule that about 180 liters per day of glomerular filtrate is formed.
The glomerular filtrate leaving Bowman's capsule enters the next portion of the nephron, the tubule. At the same time, the efferent arteriole breaks up into capillaries which come into the most intimate relationship with the tubule cells. These capillaries form a tangled network or plexus around the tubules and are, therefore, called peri-tubular capillary plexus. The tubule wall, composed of apparently stable cellular elements, appears to be in a continuous state of seething eruption with exchanges occuring between the inside of the tubule (the tubular lumen) and the peritubular capillary blood. Some materials move from tubule into the blood (tubular reabsorption); others move from blood to tubule (secretory excretion), some are formed in the tubule cells from materials in either blood or the tubular lumen (tubular synthesis). These may be reabsorbed or excreted.
Many tubules now come together into a collecting duct, still heavily invested with capillaries. A few changes are made in the collecting duct, and the urine, in its final volume and composition, is passed on to the next portion of the urinary tract. The schematic tubule just discussed was the basis of our understanding of kidney physiology for many years. Recent developments indicate that important roles must be allocated to structural peculiarities of the nephrons which were long considered of minor interest. They are not yet fully understood. The nephron is shown making a hairpin loop in a region called the loop of Henle. The cells in this loop are quite thin; they seem deficient in energy sources; yet they may play a role in the formation of the urine. The thin loop divides the tubule into two parts; the first part, the part originating at Bowman's capsule is called the proximal tubule; the second part, which eventually joins is the collecting tubule, is called the distal tubule. The anatomical relationships of the distal tubule show a very striking and suggestive peculiarity.
As the distal tubule rises from the thin loop, it bends backward to make a close contact with the afferent arteriole. The area of contact contains cellular elements of many kinds-including epithelial cells, nerve cells, and muscle cells and it has been suggested that the whole complex, named the juxtaglomerular apparatus, somehow adjusts the caliber of the afferent arteriole (and therefore the rate of glomerular filtration) to tubular activity. Thus, if so much glomerular filtrate were produced that the tubules could not modify it suitably, it can be imagined that the unsuitable urine would serve to stimulate the afferent arteriole to constrict. The smaller amount of filtrate produced could now be handled better by the tubules. This theory, though attractive, has not been proved. It has also been speculated, but not proved, that the juxta-glomerular apparatus is the source of renin (See Chapter 16).
Gross Structure of the Kidney and Urinary Tract:
 |
 |
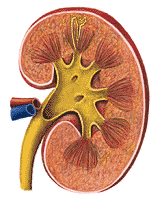
The urinary tract begins with the kidneys, which form the urine; the urine formed by the processes referred to in the last section is carried by the ureters to the urinary bladder, where it is stored until it is voided through the urethra. The anatomical relationships of the kidney, ureters and urinary bladder are shown in plates 5 and 6 of the Dissectograph. The kidneys are dorsal to the peritoneum which lines the abdominal cavity, and ventral to the spine and the muscles associated with it. As can be seen by comparing plates 1 and 7 of the Dissectograph with plates 5 and 6, the upper parts of the kidneys are closely related to the lower rib cage. In ordinary circumstances, this is useful, the ribs acting as a shield for the kidneys; sometimes, fractures of the lower ribs result in penetration of the kidney by the sharp bone fragment. This may produce disastrous hemorrhages, particularly in motorcycle accidents.
The human kidney is so characteristically shaped that the word "kidney-shaped" has become part of the language. A view of the kidney is shown in Figure 310. The whole kidney is anchored by the structures which enter it at its hilum. The large renal artery and renal vein come into the kidney here and the ureter leaves from almost the same area.
The outside of the kidney is covered by a thin fibrous capsule. This is ordinarily of no importance, but it may become important in disease when it becomes inflamed; it may become thickened and adherent to the kidney producing serious disturbances in kidney function (perinephritis).
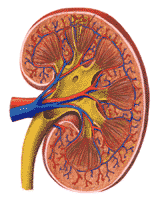
Within the capsule, the outermost layer of the kidney is called the cortex. This area, reddish brown in health contains the afferent and efferent arterioles, the glomerular capillaries, and Bowman's capsules. The juxta glomerular apparatuses, which are associated with the distal tubules, are also located here. The system of arteries and veins that feed the nephrons is overlaid in Figure 311.
Below the cortex is the renal medulla. The proximal tubules plunging downward, the thin loops, making a hairpin turn and the distal tubules returning to the afferent arteriole and then to the collecting tubules are so regularly arranged that even the naked eye can recognize a fan-like pattern of medullary rays.
Groups of nephrons come together in finger-like projections of the renal medulla; these are called papillae, which feed into the renal pelvis. The collecting ducts empty between the papillae. Urine delivered into the renal pelvis is not changed in composition or volume before delivery to the bladder. The relationship of the structures of the nephron to the macroscopic structures of the kidney are shown diagrammatically in Figure 310. The renal pelvis may be likened to a passive funnel which collects the formed urine. The ureters act as the stem of the funnel. The ureters should not, however, be considered quite inert. Their walls are muscular and they normally show contraction waves which progress from the renal pelvis to the urinary bladder.
Each ureter enters the bladder from below and behind, just off the midline (Figure 312). The empty bladder is shown in sagittal section in Figure 313. Note that the bladder roof sits on the bladder floor when the bladder is empty. As the bladder is filled, the roof is elevated, without stretching the wall. When it is quite filled (usually about 200 to 350 ml) the bladder wall is stretched and there is an urge to void.
Voiding occurs through the urethra. In women this is a short muscular tube which is guarded by internal and external sphincters- muscles which operate to close it. Its origin is at the base of the bladder (Figure 31.5). It empties outside between the labia minora of the female genitalia, above the vagina and below the clitoris. These structures are shown in Figures 316 and 317.
In the male, the urethra follows a longer course. The anatomical association with the reproductive organs, which is only incidental in the female, is of functional significance in the male.
After its beginning, which is like that of the female, the male urethra is surrounded by the prostate gland, which produces some of the seminal fluid. The same part of the urethra is entered by the ducts of the seminal vesicles, where sperm cells produced in the testicles and transmitted to the seminal vesicles by the vas deferens and seminal fluid produced by the seminal vesicles themselves are stored. The next portion of the urethra is quite short and is entered by the ducts of two small glands similar in function to the prostate. This portion of the urethra connects to the penile urethra, the terminal portion. These relationships are shown in Figure 318 and 319.
From the above, it will be seen that the male urethra may serve to conduct either seminal fluid or urine. Its role in sexual function will be considered in more detail in Chapter 27.
4. Glomerular Filtration Rate:
The process of urine formation begins with glomerular filtration, and this process is in a real sense, the heart of kidney function. The glomerular filtration rate depends on the hydrostatic pressure in the glomerular capillaries, the osmotic pressure of the plasma proteins and the pressure in Bowman's capsule. The following values may prevail in a normal person:
 |
Net effective filtration pressure: 17 mm Hg
With such an effective filtration pressure, the filtration rate might be 120 ml/min. In this case 1 mm Hg corresponds to a filtration of 4 ml/min. Consider the effects of lowering hydrostatic pressure in such a person. This might be accomplished by constriction of the afferent arteriole or dilation of the efferent arteriole or both (Figure 320). If the hydrostatic pressure were lowered 20 mm Hg, the new balance of forces would be:
Net effective filtration pressure: 5 mm Hg
The lowered effective filtration pressure would correspond to a glomerular filtration rate of 40 ml/min, only one third normal. This type of change may actually occur in shock. The kidney appears to be functionless; yet the primary disease is circulatory.
Obstructions in the lower urinary tract may raise ureteral pressure, tubular pressure and capsular pressure. Again, the change may be seen as reduced renal function; but the kidneys themselves are not at fault.
In most animals (and in human infants) the glomerular filtration rate varies with the diet and the state of hydration. High protein diets tend to increase the rate and increased hydration does the same. Adult humans (with one exception to be described below) appear to have a relatively fixed glomerular filtration rate. The reason for the difference between men and other species is not known.
The exception to the rule that adult healthy humans have a fixed glomerular filtration rate is observed in pregnancy; increases of as much as 100% are quite common at term. It is not known how these increases are brought about; it would be well worth knowing, since elevation of a depressed glomerular filtration rate may be remedial in some kidney diseases (See Part 9).
The glomerular filtration rate can be measured by measuring the excretion rate of a substance which is ultrafiltered and is not reabsorbed or excreted by the tubules. For such compounds, the rate of appearance in the urine is evidently the product of the glomerular filtration rate and the plasma concentration. If the rate of appearance in the urine and the plasma concentration are both known, the glomerular filtration rate is known.
For example, inulin, a polysaccharide, is completely ultrafilterable. It is not reabsorbed by the tubules nor is it secreted by them. If the amount appearing in the urine per minute is 125 mg and the concentration in the plasma is 1. 0 mg/ml, then it is obvious that the glomerular filtration rate was 125 ml/min. A person with disease may show similar excretion rates for inulin; but the plasma concentration may be higher, say 5.0 mg/ml. In such a person, the glomerular filtration rate is obviously 25 mg/ml (125/5). It must be noted here that the ratio excretion rate/plasma concentration will give filtration rate, if, and only if, the substance used is completely ultrafilterable and is not touched by the tubules. Very few such substances exist. Inulin, as mentioned, is one of them; sucrose is another.
5. Excretion of Glucose:
The way in which glucose is excreted shows in a readily understandable way how many substances which are reabsorbed by the tubules behave. The ability of the tubules to reabsorb glucose from the glomerular filtrate is normally limited to about 300 mg/min. For example, if the plasma concentration of glucose is 4 mg/ml and the glomerular filtration rate is 125 ml/min one would expect the filtration of 500 mg of glucose/minute. Only 300 mg can be reabsorbed; therefore 200 mg will appear per minute in the urine. When the plasma glucose is 3 mg/ml, the filtration of glucose will be 375 mg/min and 75 mg/min will escape reabsorption. The 300 mg/min difference is called the tubular maximum for glucose.
When plasma glucose falls to 2 mg/ml and only 200 mg per minute is filtered, one would expect the glucose to disappear entirely from the urine. This, is in fact, the way the dog kidney behaves. The human kidney shows some glucose appearing in the urine even when the amount filtered is less than the tubular maximum. This stems from the fact that the nephrons in man are not all equally good at glucose reabsorption. Thus some nephrons may be flooded with glucose and permit its passage into the urine while others have an unused capacity to reabsorb glucose, which cannot be transfused to those which are weak.
In normal persons, no nephrons are so weak as to be unable to reabsorb glucose at concentrations less than 1.8 mg/mi. This is the concentration at which sugar first appears in the urine-the glucose threshold. The appearance of sugar in the urine is often used as a simple screening procedure for the diagnosis of diabetes. Like any other test, it must be used intelligently. For example, the appearance of sugar in the urine may indeed result from a high blood sugar. But it can also result from a high glomerular filtration rate, diminished tubular maximum for glucose or an unusual number of nephrons very weak with respect to glucose. The last three will give a falsely positive test for diabetes. They can be collectively described as renal diabetes. This is not truly a disease and calls for no treatment, though it may cause temporary alarm.
Falsely negative urine sugar tests may occur in persons who have low glomerular filtration rates, a high tubular maximum for glucose, or a small number of nephrons weak with respect to sugar. Of these three the first is the most common; and it must always be kept in mind in the management of a diabetic. Very often, a diabetic may take the disappearance of sugar from his urine as evidence that his disease has been reversed. Usually, such a disappearance represents the superimposition of kidney disease, lowering the filtration rate, on the diabetes. The patient in such a case may be cruelly deceived by the fact that his urine has become sugar free. The outlook is usually worse than it is in diabetes alone
The kidneys show tubular reabsorption like that of glucose for many substances. Some of these substances are of value to the body, such as amino acids, some vitamins and some organic acids. Some of them, rather surprisingly, are truly waste products and tubular reabsorption of these would appear to be self-defeating. Sulfate ion and uric acid serve no useful function, yet they are reabsorbed in the tubules. These materials (and glucose) are actively reabsorbed. They may be removed fron tubular lumen against a concentration gradient-thus glucose may be present at a concentration of 0.1 mg/mi in the tubular lumen and 1.0 mg/mi in the peritubular capillary blood. Nevertheless, it will be transported from the lumen to the blood, though it would be expected to move the other way-from the area of high to that of low concentration. Active transport is not well understood. It is an energy consuming process: some active transport appears to involve high energy phosphate bonds.
Other materials are reabsorbed from the tubules passively, following concentration gradients. Urea, which will be discussed below, is one such. Other examples will be discussed.
6. Excretion of PAH:
Para-amino-hepurate (PAH), a compound not ordinarily found in the body illustrates the process of tubular excretion very well. Like glucose, this substance is actively transported; but in this case it is transported from the peritubular capillary blood into the filtrate. Like glucose, there is a maximum rate of transport for PAH. The PAH is completely removed from the blood unless the tubular maximum is exceeded. This is similar to the behavior of glucose which is almost completely removed from the tubular lumen up to the maximum filtration rate.
The behavior of PAH at levels below the tubular maximum makes it possible to use it for the determination of the renal blood flow. If, for example, the concentration of PAH in the blood is 0.01 mg/ml and if PAH is excreted in the urine at the rate of 10 mg/min, then the renal blood flow must be 1000 ml/min (10/0.01=1000). This value might be encountered in a small person. A man of normal size (5'9",170 lb) might show the following values: PAH concentration in blood 0.02 mg/ml; PAH excreted 30 mg/min; renal blood flow 1500 ml/min. The tubular maximum for PAH is about 70 mg/min; when PAH is excreted at that rate, the tubules are "swamped" and it is no longer true that PAH is completely removed from the blood. In these circumstances, the ratio of PAH excretion to blood concentration no longer defines renal blood flow.
Only a few naturally occuring substances are handled like PAH. The only one excreted as effectively as PAH is penicillin. Creatinine, one of the metabolic wastes, is excreted by the tubules in man, but the tubular maximum is very small, and probably very different from tubule to tubule (compare differences in tubular ability to reabsorb glucose). Thus, creatinine is not completely removed from the blood as it passes through the kidney and the ratio of creatinine excretion to creatinine concentration in the blood cannot be used to measure the renal blood flow.
The measurement is usually
made on plasma rather than whole
blood; whole blood works better. The ratio of excretion rate to blood
concentration is usually called clearance, and the development of modern
ideas about kidney function owes much to the introduction of the word
into the language of the kidney physiologist. The clearance concept is
less used now than in the past.
7. Excretion of Water and Sodium:
Throughout the proximal tubule,
the urine appears to have the same
osmolarity as the plasma, 305 mosm/liter. The principal solute
is sodium, present as the chloride and bicarbonate salts. There are, of course,
other solutes, such as potassium salts and glucose, but for the most part the
behavior of water is governed by the behavior of the sodium salts.
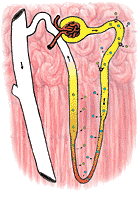 |
Sodium in the proximal tubule is actively reabsorbed. Its transport, unlike that of glucose, appears to be limited by a concentration gradient. Thus, when sodium is reabsorbed, the lowering of sodium concentration within the tubule eventually disables the transfer mechanism (Figure 321). However, the reabsorption of sodium, by lowering the osmolarity of the tubular fluid, creates a strong osmotic tendency for water to follow the osmolar gradient: in effect sodium transport obligates the movement of water throughout the length of the proximal tubule. It seems probable that about 85% of the sodium (and water) of the glomerular filtrate are reabsorbed in the proximal tubule.
The distal tubule is apparently capable of transferring sodium against large concentration gradients. Further, it is able to restrain the movement of water, so that the urine in the distal tubule becomes distinctly lower in osmolarity than the blood. Conversely, the transferred sodium tends to increase the osmolarity on the outside of the distal tubule. This has very little effect on the cortical portions of the kidney, which have a very rich blood supply. The transferred sodium is immediately carried away. The medullary portions of the kidney, however, have a very poor blood supply; sodium transferred out of the distal tubule increases the osmolarity of these areas vary greatly. The less well supplied the area is with blood, the greater will be the effect of the transferred sodium on osmolarity.
This process results in high osmolarity of the medullary areas which in man may reach three or four times the osmolarity of the plasma. In some desert animals, particularly the kangaroo rat, parts of the renal medulla may reach an osmolarity ten times that of the plasma.
The thin loop, which passes through the medullary area made hyperosmolar by the activities of the distal tubule is probably in concentration equilibrium with that area, losing water to it and gaining sodium from it. The distal tubule therefore receives a small volume of a strongly hyperosmolar urine, upon which it performs the processes already described. In the end, the distal tubule, containing urine low in osmolarity and somewhat reduced in volume, proceeds toward the renal pelvis through a hyperosmolar medulla. Here an extraordinary event may occur.
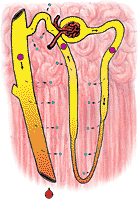 |
fig 322. Urine and Medulla Osmolarity in a Dehydrated Nephron |
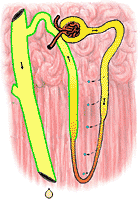 |
fig 323. Urine and Medulla Osmolarity in an Overhydrated Nephron |
In an animal which has been dehydrated, the terminal portions of the distal tubule are made permeable to water, which is now free to follow its osmolar gradient. Since the distal tubule courses through a highly hperosmolar area, large quantities of water may be lost from the urine. The urine which issues into the renal pelvis is as hyperosmolar as the renal medulla and in man may be reduced in volume to as little as 0.1 ml/min. In the kangaroo rat (which it will be recalled had an extremely hyperosmolar medulla) the urine may approach an osmolarity of 3000 mosm/l with consequent savings in water.
In a well hydrated animal, on the other hand, the distal tubules remain quite impermeable to water. The urine does not become equilibrated with the renal medulla at all; its volume is large and its osmolarity is small. The difference between the well hydrated and poorly hydrated animal depends on the presence of a hormone from the posterior lobe of the pituitary gland. This hormone, once called vasopressin (because it causes small blood vessels to constrict) is more properly called the antiduretic hormone (ADH). In general, ADH release is inhibited when the blood of the animal has an osmolarity below 305 mosm/l. Its release is excited when osmolarity increases. When released, this hormone acts to increase the permeability of the distal tubule to water; in its absence, the tubule's impermeability to water is preserved. The consequence is obvious: hyperosmolarity of the body fluids (usually a result of dehydration) leads to water conservation; hypo-osmolarity (usually a result of overhydration) leads to the elimination of large volumes of hypo-osmolar urine.
Figure 322 shows the osmolarity of various portions of the nephron in an animal which is hyperosmolar. Figure 323 shows the same for a hypo-osmolar animal.
It should be stressed here that the secretion of ADH depends on the osmolarity of the fluid presented to the hypothalamic center which governs its release and not on the actual hydration of the animal. For example, a man given a liter of a strong salt solution, say 610 mosm/l by mouth or vein responds inappropriately as far as volume is concerned. The extra liter of fluid increases the osmolarity of body fluids rather than decreasing it and ADH is released, preserving the increase in volume. Similarly, a person put on a low salt diet, which tends to reduce the osmolarity of his body fluids, may as a result of that fact alone respond by reducing ADH secretion. This will result in fluid loss even though there has been no unusual intake of fluid.
The hypothalamic center which controls the release of ADH is controlled by nervous influences as well as by osmolarity. Exercise and emotion both appear to excite it to cause production of ADH. Sleep, by an unknown mechanism, appears to do the same in the adult, though not in the infant. Alcohol appears to raise the level of osmolarity which will excite ADH production. Alcoholic excesses may, in consequence, cause the production of large volumes of urine of low osmolarity, even when the body fluids are hyperosmolar. It is possible that some of the miseries which follow such excesses are associated with disturbed regulation of fluid osmolarity, as well as acetaldehyde production (Chapter 21). This would suggest that water might be beneficial in stubborn hangovers. This remedy, though unproved, has the virtue that it is very cheap.
The total amount of sodium excreted by the kidney is unbelievably small in relation to the amount filtered. Expressed as sodium chloride, about 1.6 kg is filtered per day. Of this, usually no more than 10 gm is excreted, less than 0.7%. There seems to be no doubt at all that no less than 85% of the filtered sodium is recovered in the tubules without being "exchanged" for anything just as glucose is. Furthermore, it is almost impossible to alter this part of sodium reabsorption. It appears that the kidney is so constructed that it must reabsorb at least this much sodium without any external control.
Part of the remainder of sodium reabsorption may be under the control of the adrenal cortex, through a hormone called aldosterone. This hormone, which will be discussed again in Chapter 25, is released from the adrenal cortex when the body sodium is low. It is believed to act on the distal tubule, promoting both sodium reabsorption and potassium excretion. The exact nature of the stimulus for aldosterone production is not known; but it appears in greater quantities when the extracellular fluid volume is decreased; and its formation is suppressed when extracellular fluid volume is increased. It may, therefore, be considered to regulate the total amount of sodium in the organism, while the antiduretic hormone regulates its concentration.
8. Formation of Ammonia and Carbonic Acid:
Ammonia is made by the kidney from the amino acid
glutamine, and is usually present in excess. This makes it possible for
the kidney to reabsorb sodium without reabsorbing the anion associated
with it. For example, during protein breakdown, the sulfur of the protein
is degraded to sulfuric acid. This strong acid which is produced in the
liver is immediately neutralized by NaHCO3, present in the
circulating blood. The reaction may be written as follows:
H2SO4 + 2NaHCO3 --> Na2SO4
+ H2CO3
The carbonic acid (H2C03) is broken down to water and CO2,the latter being eliminated in the lungs. However, the cost of the neutralization is paid by depletion of the body's bicarbonate reserves. This depletion would have serious consequences (Chapter 23) if the kidney did not correct it. It does so in the following manner: The sodium sulfate formed in the reaction comes to the kidney where a part of it undergoes filtration. In the glomerular filtrate, the sodium and sulfate ions are, of course, separate. They are not, however, independent. For convenience, they will be described as if they were present in molecular form as Na2SO4.
The reabsorption of sodium sulfate
involves as a first step the
formation of carbonic acid by the kidneys. The formation of carbonic acid occurs
readily when carbon dioxide, water and the enzyme carbonic anhydrase are
all present.
H2O + CO2 (Carbonic Anhydrase)--> H2CO3
The kidney's carbonic anhydrase concentration is in fact, very high.
The next step involves the reaction of carbonic
acid and sodium sulfate:
H2C03 + Na2SO4
<--> H2SO4 + 2NaHCO3
This reaction will always proceed to the left unless one or both of the endproducts on the right are removed.
The availability of ammonia in the kidney makes
removal of the sulfuric acid possible:
2NH3 + H2S04 -->
(NH4)2S04
Sodium carbonate, the other end product of the reaction, is removed by reabsorption.
Note that the two reactions performed by the kidney, the synthesis of ammonia and bicarbonate, result in the excretion of ammonium sulfate without significantly changing the body's reserve of either sodium or bicarbonate. The kidney, in this sense, acts to correct the defect produced by the liver's production of acid.
It should be noted here that many
metabolic acids are produced in the
body, not only by the kidney, but also by other organs. Through its
synthesis of carbonic acid and ammonia, the kidney tends to correct
the acidosis so produced.
9. Excretion of Urea:
Most nitrogen taken by mouth is
converted in the liver to urea, a simple
organic molecule which is usually eliminated quite readily by the
kidneys. Urea enters the glomerular filtrate at the same concentration as exists
in plasma. As sodium and water are reabsorbed in the proximal tubule, the
urea concentration tends to rise. This leads to a diffusion of some urea
back into the blood since the proximal tubules are somewhat permeable to
urea.
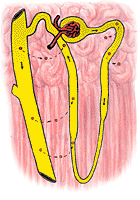 |
The distal tubule is much less permeable to urea particularly when ADH is absent. When the urine flow is large, the amount of urea in the urine is about 70% of the amount filtered. At low urine flows, virtually none of the urea filtered may appear in the urine. The reabsorption of filtered urea into the bloodstream during low urine flow is illustrated in Figure 324.
It may seem odd that a substance like urea which is so obviously destined for excretion should be eliminated by such an ineffective mechanism. It should, however, be noted that urea by itself has no ill effects. The term uremia often used to describe the condition of persons in kidney failure is correct in the sense that such persons do, in fact, have high blood urea. Their difficulties do not, however, stem from the high level of urea, but rather from loss of other functions of the kidney.
10. Diseases of the Urinary Tract:
The nature of the forces which operate to produce the glomerular filtrate is such that diseases elsewhere in the body are sometimes made manifest as disorders of kidney function. For example, in men, the prostate gland may hypertrophy obstructing the bladder outlet. This brings about an increase in urinary pressure, which may be communicated to Bowman's capsule. These pressure changes may reduce the glomerular filtration rate to a point where the kidney seems to be virtually non-functional. The kidney may, nevertheless be quite normal, and may resume its function as soon as the obstruction is relieved.
A very dramatic type of renal failure in which the kidneys are the victims rather than the cause of the illness is that seen after hemorrhage or shock. The low arterial pressure usually associated with these conditions compromises the ability of the kidney to form glomerular filtrate. In addition, the barostatic reflexes often operate to cause afferent arteriolar constriction. The ability of the kidney to form a glomerular filtrate is, thus, doubly impaired; and, in many cases, an otherwise non-fatal injury may have a fatal outcome because of kidney failure.
Something of the same kind is often seen in chronic congestive heart failure. The damaged heart with a diminished cardiac output cannot maintain normal arterial pressure without the assistance of the barostatic reflexes. When these are invoked, the afferent arteriole may be so constricted as to reduce the formation of glomerular filtrate; however, it rarely stops altogether. The picture usually seen is one of glomerulo-tubular imbalance. The tubules, essentially normal, reabsorb as usual; the glomerular filtration rate may fall below the tubular ability to reabsorb, in which case elimination of certain substances is gravely impaired. The most striking example has to do with the excretion of sodium. Slight reductions in the glomerular filtration rate, not quite compensated by similar reductions in the tubular reabsorption of sodium may result in the total failure of sodium excretion. With this failure, the body fluids become hyperosmolar and water too is retained. The accumulated sodium and water make an extracellular edema. The edema fluid may accumulate behind the right heart, or behind the left heart, or both (See also Chapter 16).
This view, which attributes the accumulation of edema fluid to excretory failure on the part of the kidney towards sodium, has made possible the development of some truly remarkable methods for the treatment of heart failure. All of them are based on the idea that the elimination of sodium from the body will have a favorable effect on the disease.
In some cases, the cardiac patient with edema will be relieved of symptoms simply by reducing his intake of sodium. Usually, however, it is necessary to remove so much of the normal salt intake of the diet that food becomes quite unpleasant; there is the further difficulty that many prepared foods (bread, for example) have a fairly high salt content.
A much more effective means of correcting glomerulo-tubular imbalance involves interfering with tubular function. This can be done by the use of general tubular depressants (such as mercury); or by the administration of drugs which specifically impair sodium reabsorption.
One such drug is the carbonic
anhydrase inhibitor acetozolamide. This drug, which is concentrated in
the kidney, reduces the rate of the reaction:
H2O + CO2 <--> H2CO3
which is ordinarily facilitated by carbonic anhydrase.
An example of the use of carbonic acid (together with ammonia) in the reabsorption of sodium sulfate was given above (Part 7). Carbonic acid also seems to be involved in the reabsorption of sodium bicarbonate particularly from the proximal tubule, where most of its is ordinarily reabsorbed. The distal tubule is, however, less affected.
The usual consequence of giving a carbonic anhydrase inhibitor is the rapid elimination of large quantities of sodium and bicarbonate. Along with these, there is a copious output of water. This water is presumably that which escaped proximal reabsorption because it was not osmotically obligated to a high concentration of reabsorbed sodium. (Recall that in the proximal tubule water follows sodium, maintaining normal osmolarity; when sodium is rejected, water will also be rejected).
The fact that carbonic anhydrase nhibitors cause the loss of sodium bicarbonate tends to place limitations on their use. It will be shown in the next chapter that these losses of bicarbonate may result in very grave changes in the extracellular fluid. As part of an effort to find a more effective carbonic anhydrase inhibitor, a drug was synthesized in 1957 which proved to have impair the absorption of sodium chloride but not sodium bicarbonate. This drug, which was subsequently found to be almost completely ineffectual as a carbonic anhydrase inhibitor, is called chlorothiazide. Its mechanism of action is unknown at the present time, though it is being intensively investigated. Unlike acetazolamide, which by favoring the elimination of NaHCO3 produces serious alterations in the composition of extracellular fluid, chlorothiazide simply reduces the volume of the extracellular fluid without significant change in composition. The drug has only one serious side-effect: it appears to impair the reabsorption of potassium ion as well as sodium. This can usually be controlled easily simply by taking extra potassium chloride by mouth. For reasons which are not understood, this drug also seems to be effective in the management of high blood pressure.
Other drugs are available for the correction of glomerulo--tubular imbalance in the patient with chronic congestive heart failure; chlorothiazide and drugs like it appear to be more effective than any others; they will not be discussed here. An excellent review of these drugs and their physiology of action has recently appeared (See New England J. Med. under Secondary References).
At one time, the kidney was thought to cause essential hypertension. There are certainly many changes in kidney function, but it now appears probable that the kidney is the victim of the disease process rather than the causative organ. In general persons with hypertension tend to have diminished blood flow to the kidneys and their glomerular filtration rates tend to be low. These changes are, however, largely reversed by drugs which prevent the generalized vasoconstriction characteristic of hypertension, and should be considered secondary to that vasoconstriction.
Bright's Disease (glomerulonephritis) is a true disease of the kidneys. Classically, it follows a rather regular pattern; in actual practice the sequence is not always followed.
The disease usually follows a streptococcal infection elsewhere in the body. Recovery may seem to be progressing uneventfully, when, rather suddenly, there is general evidence of capillary damage throughout the body, but especially in the kidneys. In this phase (acute glomerulonephritis) the urine may contain red cells and proteins and it may be much diminished in volume. It appears as if the glomerular capillaries have become leaky to red cells and proteins; at the same time there seems to be afferent arteriolar constriction. The whole process is believed to be due to an allergic response (Chapter 32) of the kidney to the toxins produced by the bacteria. Some persons so afflicted die; most recover completely; a few have a long course marked by progressive degeneration of the kidney. The organ, initially allergic to streptococcal toxins, may become allergic to itself and so destroy itself.
Some persons who recover from the acute phase of glomerulonephritis may continue to lose proteins in their urine. This protein loss may be so great as to lower the colloid osmotic pressure of the plasma proteins and a generalized edema may develop. This is spoken of as the nephrotic phase of glomerulonephritis. In this phase the kidney functions are all reasonably normal, except for the inability of the kidney to conserve proteins; the edema of the patient is easily managed by administration of plasma proteins from time to time; and, on the whole, the patient is not very sick. Many patients live out their lives in this phase; a few recover entirely.
Some proceed downhill. Damaged nephrons become scarred and non-functional; new nephrons, not previously involved, become diseased and are eventually destroyed. There is a tendency for blood pressure to rise, sometimes drastically; as kidney tissue is lost, a little at a time, the glomerular filtration rate goes down. The ability of the kidneys to produce ammonia and carbonic acid may be gravely damaged. At the same time the ability to regulate water, sodium and potassium and osmolarity may be lost. Eventually, death occurs. Although blood urea is usually quite high by the time of death, it is not the high urea which kills, so much as the loss of other kidney functions which are reflected in the level of blood urea. Persons who die in this phase show atrophic shrunken kidneys with a bewildering variety of degenerative and destructive changes in all the nephrons. These people should always be considered for transplantation or artificial kidneys. The problems of transplantation will be considered in Chapter 31. The requirements of artificial kidneys will be taken up in the next chapter.
Continue to Chapter 23.