Single Wall Carbon
Nanotubes
Alignment and Deposition
A
one-step process of solubilization of single wall carbon nanotubes
(SWCNT) in
an organic solvent has enabled us to polarize them asymmetrically in a
dc
electric field. Quaternary ammonium ion-capped SWCNTs readily suspend
in
organic solvents; under the influence of a dc electric field they
assemble as
stretched bundles anchored on the positive electrode. At low dc applied
field (~
40V) all the SWCNT from the suspension are deposited on the electrode,
thus providing
a simple methodology to design robust SWCNT films.
At higher applied voltages (>100V), the
SWCNT bundles stretch out into the solution and orient themselves
perpendicular
to the electrode surface.
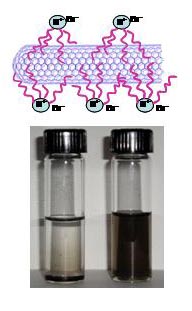
Solubilization
of SWCNT in Organic Solvents
Purified
SWCNTs synthesized by electric arc method (from SES Research ) were
solubilized
by mixing with tetraoctylammonium bromide (TOAB) in THF (10 mg SWCNT
and 0.13 g
TOAB in 25 ml THF). Sonication of the
mixture for 20-30 minutes yields a stable dark suspension. We also
solubilized SWCNT
in TOAB/THF obtained from another source Nanonics. The black suspension
was
centrifuged at 10,000 rpm for approximately 10 minutes. The clear
supernatant
liquid containing unbound TOAB was discarded. This procedure was
repeated and
the final centrifugate, after removing the solvent was dried. The
repeated
washing and centrifuging procedure allowed us to discard any unbound
TOAB from
the SWCNT material. The dried material
consisting of TOAB bound SWCNT was readily suspendable in organic
solvents. Typically TOAB bound SWCNT
was resuspended in 25 ml THF and sonicated for 10-15 minutes. Based on
weight
gain, capping of 1 mg TOAB per 10 mg of SWCNT is estimated. The
quaternary ammonium salt, TOAB
allows solubilization by binding to the SWCNT
through hydrophobic interactions of its
alkyl chains.
Electrophoretic
Deposition of SWCNT film on electrode surfaces
The electrophoresis is carried out in a 1 cm
quartz cuvette. The
SWCNT solution is transferred into the electrophoretic
cell. Two
optically transparent electrodes (conducting glass slides, cut 9
mm x 5 cm, to
fit into the cell)
were kept parallel to each other (~5
mm apart) in an electrophoretic cell.
When a dc voltage of ~ 40 V was applied, carbon nanotubes
slowly move from the suspension towards the positive electrode.
Continued
application of dc voltage for 1-2 minutes results in the deposition of
SWCNT
film on the electrode surface. The thickness of SWCNT films can be increased by
increasing the time of
electrophoretic deposition. These films are quite robust and are
suitable for
electrochemical, fuel cell, catalytic and photoelectrochemical
applications.
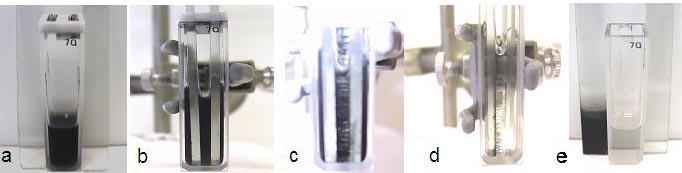 Stages of electrophoretic deposition:
(a) SWCNT suspension in the cuvette, (b) insertion of
electrodes, (c) Immediately after application of dc
field (d) end of deposition cycle, and (e) conducting
glass electrode with SWCNT film and colorless solution in the cell.
Stages of electrophoretic deposition:
(a) SWCNT suspension in the cuvette, (b) insertion of
electrodes, (c) Immediately after application of dc
field (d) end of deposition cycle, and (e) conducting
glass electrode with SWCNT film and colorless solution in the cell.
G. Girishkumar; K. Vinodgoapal, Kamat, P. V. SWCNT Films for methanol
oxidation, J. Phys. Chem. B 2004, 108, in press.
Macroscopic
Alignment in dc field
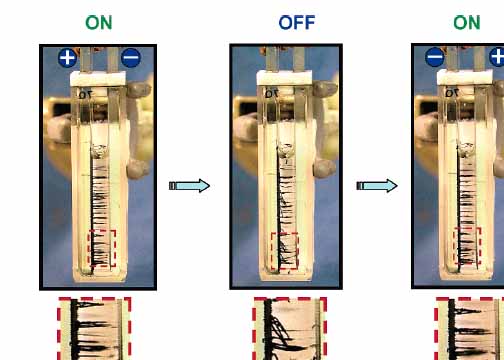 The
electrophoresis set up as described above can be used for alignment
experiment. When dc voltage of >100 V is applied between
the two conducting glass
electrodes, the nanotubes instead of undergoing deposition, assembled
into
linear bundles extending across the space between the two electrodes.
These bundles
are well separated and aligned perpendicular to the electrode surface.
When the dc field is turned off, the aligned nanotubes
quickly bend downwards. When the field is restored the tubes align
again in the
horizontal direction. The sequence in Figure shows the reproducibility
of attaining similar
alignment during ON periods of dc field. The
alignment of SWCNT bundles in a dc electric field and their response to
repeated
ON-OFF-ON cycles can
also be seen in the Movie.
The
electrophoresis set up as described above can be used for alignment
experiment. When dc voltage of >100 V is applied between
the two conducting glass
electrodes, the nanotubes instead of undergoing deposition, assembled
into
linear bundles extending across the space between the two electrodes.
These bundles
are well separated and aligned perpendicular to the electrode surface.
When the dc field is turned off, the aligned nanotubes
quickly bend downwards. When the field is restored the tubes align
again in the
horizontal direction. The sequence in Figure shows the reproducibility
of attaining similar
alignment during ON periods of dc field. The
alignment of SWCNT bundles in a dc electric field and their response to
repeated
ON-OFF-ON cycles can
also be seen in the Movie.
Kamat, P. V., Thomas, K. G., Barazzouk, S., Girishkumar, G.,
Vinodgopal, K. and Meisel, D., Self-Assembled Linear Bundles of Single
Wall Carbon Nanotubes and Their Alignment and Deposition as a Film in a
DC-Field. J.
Am. Chem. Soc., 2004, 126, 10757-10762.


 Stages of electrophoretic deposition:
(a) SWCNT suspension in the cuvette, (b) insertion of
electrodes, (c) Immediately after application of dc
field (d) end of deposition cycle, and (e) conducting
glass electrode with SWCNT film and colorless solution in the cell.
Stages of electrophoretic deposition:
(a) SWCNT suspension in the cuvette, (b) insertion of
electrodes, (c) Immediately after application of dc
field (d) end of deposition cycle, and (e) conducting
glass electrode with SWCNT film and colorless solution in the cell. The
electrophoresis set up as described above can be used for alignment
experiment. When dc voltage of >100 V is applied between
the two conducting glass
electrodes, the nanotubes instead of undergoing deposition, assembled
into
linear bundles extending across the space between the two electrodes.
These bundles
are well separated and aligned perpendicular to the electrode surface.
When the dc field is turned off, the aligned nanotubes
quickly bend downwards. When the field is restored the tubes align
again in the
horizontal direction. The sequence in Figure shows the reproducibility
of attaining similar
alignment during ON periods of dc field. The
alignment of SWCNT bundles in a dc electric field and their response to
repeated
The
electrophoresis set up as described above can be used for alignment
experiment. When dc voltage of >100 V is applied between
the two conducting glass
electrodes, the nanotubes instead of undergoing deposition, assembled
into
linear bundles extending across the space between the two electrodes.
These bundles
are well separated and aligned perpendicular to the electrode surface.
When the dc field is turned off, the aligned nanotubes
quickly bend downwards. When the field is restored the tubes align
again in the
horizontal direction. The sequence in Figure shows the reproducibility
of attaining similar
alignment during ON periods of dc field. The
alignment of SWCNT bundles in a dc electric field and their response to
repeated