Unit V - Respiration
Chapter 18
Movements of the Respiratory Gases
1. Gas Laws:
The movements of the respiratory gases are in part determined by the gas laws of physics and chemistry. As is the case in many biological systems, the laws must be modified to take account of the greater complexity of the biological system. Nevertheless, the laws are of sufficient applicability to warrant a brief description here.
a. Volume of a gas:
The volume of a gas depends on three things:
(1) The pressure exerted against the gas. Gas volume decreases as pressure increases and vice versa.
(2) The absolute temperature of the gas. As the temperature increases, the volume of the gas increases if the pressure stays constant. If the volume stays constant, the pressure increases as the temperature increases.
(3) The amount of the gas. This is measured in terms of the number of molecules, not the weight. For example, 2 grams of hydrogen (H2) fills the same volume as 222 grams of radon if the pressure and volume are the same in both cases.
These relationships are usually summarized in the general gas law:
PV = nRT
The value of R is 62,400 when pressures, volumes, and temperatures are given in the indicated units.
A few examples will be used to illustrate the gas law:
As pressure goes down, volume goes up. This may have been experienced by persons who have flown in an airplane while they have a cold. In some colds, the Eustachian tubes are blocked. As the plane gains altitude and cabin pressure falls the volume of the middle ear tends to increase. The expansion of this volume stretches the ear drum and may lead to earache.
As temperature increases, volume or pressure increases. The most familiar example is the effect of driving on tire pressure. During driving, the tires heat up; at high speeds they may become untouchably hot. A good driver, anticipating a long journey, will inflate his tires somewhat less than the recommended driving pressure. Another example had to do with the paranasal sinuses. During a cold with fever, the drainage of these sinuses may be blocked. In these circumstances, increases in body temperature bring about increases in pressure within the sinus, often leading to headache.
The amount of the gas is measured in terms of the number of molecules, not the weight. Helium (weight 4 grams per mole) is sometimes substituted for nitrogen (weight 28 g / mole) in the air supplied to patients in respiratory distress. This is usually done to make the flow of the air easier. Only 1/7 of the weight of nitrogen needs to be added as helium for the respired gas to have the same volume as normal air.
b. Partial pressure of a gas:
In a mixture of gases, each gas can be considered as if it were quite alone from the standpoint of the pressure it exerts. A liter of gas composed of 800 ml of nitrogen and 200 ml of oxygen at pressure of 1000 mm Hg exerts a pressure of 800 mm Hg nitrogen and 200 mm Hg. The total pressure is 1000 mm Hg, but the pressure due to oxygen is only 200 mm Hg. If 200 ml of oxygen were introduced into an evacuated one liter container, the behavior of the oxygen would be identical to its behavior in the mixture.
An interesting example of this comes from astronautics. A space capsule designed to simulate normal air with respect to oxygen can operate at a total pressure of 152 mm Hg, provided it is filled with 100% oxygen, at least so far as delivering oxygen to the astronaut is concerned. In high altitude flying, particularly in military flights, the atmospheric pressure may be as low as 200 mm Hg. This atmosphere is one fifth oxygen (by moles), just as at sea level. Thus the partial pressure of oxygen is only 40 mm Hg, a level incompatible with life.
The partial pressure of air in the normal, sea level, atmosphere is 152 mm Hg. The partial pressure of air in the lungs is much less--about 109 mm Hg. This is due to the fact that the gases from the outside coming into the lung mix with gases from the blood which are leaving the lung. Alveolar air is, therefore, a mixture whose composition lies between that of the atmosphere breathed and the gases delivered by the blood.
The partial pressure of a gas determines its solubility in a liquid and a liquid saturated with gas at a given partial pressure has the same partial pressure with respect to the gas as the gas itself.
The first part of the statement implies that the solubility of a gas in a liquid is directly proportional to its partial pressure. This is true only if the gas does not enter into chemical combination with the liquid or element of it. For example, the solubility of oxygen in blood is much greater than would be expected from its partial pressure and its solubility in water; the difference stems from the fact that oxygen enters into chemical combination with the hemoglobin of blood. When chemical combinations do not occur, as is the case with oxygen solubility in plasma, the proportionality between partial pressure and solubility is quite direct. For example, at a partial pressure of 100 mm Hg, 3.0 ml of oxygen are carried by one liter of plasma (compared to 200 ml carried by whole blood). When alveolar oxygen partial pressure is increased to, say, 500 mm Hg, the solubility also rises five times to 15 ml per liter. It has recently become possible through the development of hyperbaric chambers to increase the partial pressure of oxygen in the alveoli to 5000 mm Hg, which raises the solubility in the plasma to 125 ml/liter. This amount of oxygen in the plasma will support life and has recently been used for this purpose in the case of a person with a very severe anemia who refused a blood transfusion.
The statement that liquids in equilibrium with a gas at a given partial pressure have a partial pressure proportional to the gas may seem odd, but nevertheless, it is true. Thus raindrops, though they contain very little oxygen, have a partial pressure of oxygen which is the same as that of the air, 152 mm Hg. Mercury exposed to air dissolves virtually no oxygen, but its partial pressure with respect to oxygen is the same as that of the air.
The statement may seem less odd if the partial pressure of a gas in a liquid is viewed as the force exerted by the molecules of the gas escaping from the liquid. Obviously, a liquid which can dissolve only a little of a gas cannot really hold it. The small quantity of dissolved gas, may exert a very high pressure in escaping from the liquid.
The solubility of a gas in a liquid tends to be reduced as the temperature increases.
A familiar example of this is seen when water is heated but not boiled. The bubbles of gas which form at temperatures well below the boiling point represent gases which were soluble in the water. An illustration of physiological interest has to do with the greater solubility of oxygen in the blood of the lungs, which are cool, compared to that of the tissues, which are quite warm. This will be considered in more detail in Part 2.
At temperatures above the boiling point, water exists entirely as a gas, a fact which is appreciated by everyone. It is less well known that water exerts a significant partial pressure at any temperature. Even ice at 0 oC has a partial pressure of water of 4.6 mm Hg. At body temperatures, the vapor pressure of water is 47 mm Hg. This high partial pressure of water in the respiratory passageway is uncontrollable. Because of it, the other respiratory gases exert a rather smaller partial pressure than would be expected. It is as if some 6. 4% of the lungs and respiratory passages were filled by an inert gas at sea level. It may be mentioned that at 63,000 feet, the atmospheric pressure is only 47 mm Hg. The partial pressure of water, which is quite independent of the outside pressure and depends only on body temperature, has the same value. This means that the respiratory passages and lungs contain virtually no gases other than water. At this altitude oxygen breathing cannot introduce oxygen into the lungs at all. To a smaller extent this is true at lower altitudes. Thus at 50,000 feet, where the barometric pressure is 87 mm Hg, breathing 100% oxygen results in an alveolar oxygen tension of only 16 mm Hg. Water vapor (47 mm Hg) and carbon dioxide and nitrogen returning from the tissues (24 mm Hg) make up the balance.
2. Alveolar Ventilation:
The pulmonary arterial blood delivers water, carbon dioxide and nitrogen to the alveoli. The water and carbon dioxide are eliminated through the airway and oxygen and nitrogen are taken in. The result of these processes is that the composition of the alveolar air is somewhere between the composition of the inspired air and that of the pulmonary arterial blood. The blood which leaves the lungs for the left heart and body is basically in equilibrium with the alveolar air. It thus becomes of the utmost importance to determine how the alveolar air is changed by respiratory activity.
Two qualitative statements will be made first:
(1) The composition of the alveolar air will approach that of the pulmonary arterial blood during breath holding.
(2) Increasing the depth and frequency of breathing will make alveolar air much like the outside air.
Between these limits, the composition of the alveolar air can be described in terms of some simple equations. These equations are presented for students who are interested. Others, particularly in elementary courses, may be interested only in the conclusions.
The volume of air expired in the course of one minute consists of n breaths each of volume V. Each breath contains some alveolar air (volume = a) and some dead space air (volume d). The amount of air breathed per minute can be written nV. This is equal to the sum of na + nd as is seen in equation (1):
(1) nV = na + nd
The amount of carbon dioxide eliminated per minute, ECO2, can be given as the product of the volume of alveolar air and the molar fraction of alveolar air which is carbon dioxide (FCO2). This is described in equation (2):
(2) ECO2 = naFCO2
The uptake of oxygen / min may be given in terms of the mole fractional difference between the alveolar air and the inspired air as described in equation (3):
(3) UO2 = na (FO2air - FO2alveolar air)
If the dead space is known, this system of equations can be completely solved. For example, if the dead space is 150 ml, the volume of each breath is 400 ml, the number of breaths / minute is 16, the minute volume of CO2 is 200 ml, and the minute volume of O2 is 250 ml, we can derive the following:
(1) nV = na + nd
16 x 400 = 16 x a +
16 x 150
a = 250 ml
Substituting this value in equation (2) we obtain:
(2) ECO2 =
naFCO2
200 = 16 x 250 x
FCO2
FCO2 = .05 = 5 %
Using 0.21 as the value for the mole fraction of O2 in the inspired air, equation (3) gives:
(3) UO2 =
na (FO2air -
FO2alveolar air)
250 = 16 x 250 x (0.21 - FO2alveolar air)
FO2alveolar air
= 0.143
so the mole fraction of oxygen in the alveolar air is 0.143.
Assuming that barometric pressure of alveolar air is 760 mm, the partial pressure of CO2 is 38mm Hg, while that for oxygen is 109mm Hg. These are very close to the true values.
The effects of increasing respiratory activity on the levels of the respiratory gases are interesting. Suppose that without other changes, the frequency of respiration was doubled. According to equation (1):
(1) nV = na + nd
32 x 400 = 32 x a +
32 x 150
a = 250 ml
The alveolar ventilation per breath would remain the same.
According to equation (2):
(2) ECO2 =
naFCO2
200 = 32 x 250 x
FCO2
FCO2 = .025 = 2.5 %
According to equation (3):
(3) UO2 =
na (FO2air -
FO2alveolar air)
250 = 32 x 250 x (0.21 - FO2alveolar air)
FO2alveolar air
= 0.177
so the mole fraction of oxygen in the alveolar air is 0.177.
These mole fractions would represent partial pressures of 19 mm Hg for CO2 and 135 mm Hg for oxygen.
The student may find that precisely the same effect is brought about by increasing the depth of each breath to 650 ml and breathing 16 times / minute.
Doubling respiratory frequency without changing the volume per breath is much less effective in changing alveolar air than doubling the depth of respiration. This is easily seen from the last two examples. In increasing frequency to 32 breaths / min, twice normal, the alveolar air was changed as much as it was in increasing depth to 650 mI / min at a frequency of 16. The increased total ventilation was 1.63 times normal. The difference between the two modes of respiration results from the existence of the dead space.
If we assume that no change in respiratory activity occurs in activity, doubling the utilization of oxygen and the production of carbon dioxide would result in a partial pressure of CO2 of 78 mm Hg and 63 mm Hg of oxygen. It will be shown in Chapter 19 that the continuance of respiratory activity is to a large extent dependent on the level of these two gases, so that rises in carbon dioxide and falls in oxygen partial pressure lead to increased respiratory activity both in rate and frequency.
Breath-holding has relatively minor effects on the composition of the alveolar air. The equations used above are not applicable for breath-holding since they describe steady states. If the breath is held for 5 seconds, and if CO2 and O2 are transferred at the usual rates (200 ml / min for CO2, 250 ml / min for O2), the alveolar air, about 5 liters in volume containing 250 ml of CO2, will rise to almost 300 ml of CO2, corresponding to a partial pressure of 46 mm Hg. At the same time it will lose at most 60 ml of oxygen. Since it contains some 700 ml of oxygen, the reduction in the partial pressure of O2 will be less than 10 mm Hg. The figures given are maximal; actually CO2 delivery and oxygen removal occur at a slower rate during breath holding than in normal breathing because the partial pressure difference between blood and alveolar air is reduced during breath holding.
From the calculations above, it should be evident that the process of alveolar ventilation results in the exposure of the pulmonary blood to an atmosphere much less like that of the outside air than it is like the internal environment of the cells. The table which follows shows this:
| pp CO2 | pp O2 | |
| Mixed Venous | 45 mm Hg | 50 mm Hg |
| Alveolar | 40 mm Hg | 110 mm Hg |
| Arterial | 40 mm Hg | 105 mm Hg |
| Outside | 38 mm Hg | 109 mm Hg |
Alveolar ventilation is effective because it is continuous; but it is less efficient than a system in which exchanges occur directly with the outside. It becomes more efficient as the depth of respiration is increased, and it is less efficient during rapid shallow breathing which ventilates primarily the dead space rather than the alveoli.
 |
 |
3. Exchanges of Oxygen:
Oxygen is transported in a chemical combination with hemoglobin in the red cell. Two steps are necessary for adequate oxygen transportation. First, it must be exposed to a high partial pressure in the lungs. Second, it must be exposed to a low partial pressure of oxygen in the tissues which are to use it. It goes without saying that the blood must have adequate amounts of hemoglobin and that it must circulate at adequate rates.
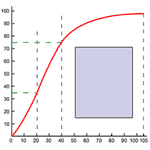
There is, however, a peculiarity in the oxygen hemoglobin-combination. The combination is nearly complete at surprisingly low partial pressures--90% complete even at 65 mm Hg partial pressure, such as exists in many tissues. Thus 160 grams of hemoglobin can carry 2 liters of oxygen when it is exposed to pulmonary partial pressures of 100 mm Hg. In a tissue with a partial pressure of 65 mm, the same hemoglobin concentration would still carry 1800 liters of oxygen. The strength of the combination is such that only 10% of the carrying capacity is used in delivery. It is as if a truck which could carry 20 tons could only unload 2 tons at its destination and returned to its origin still carrying 18 tons of the original load. The relationship between hemoglobin carrying capacity and partial pressure in vitro is shown in Figure 273.
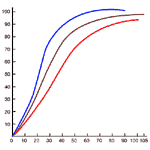
Actually the situation is not nearly so bad. In the tissues, many circumstances act together to assist in the unloading of oxygen. Carbon dioxide, present in active tissues, appears to break the hemoglobin-oxygen bond and to release the oxygen. The effect of carbon dioxide on the breakdown of the hemoglobin-oxygen complex is illustrated in Figure 274. Many other circumstances which exist in active tissue have the same effect. Heat and acid, both characteristic of activity reduce the carrying capacity of hemoglobin for oxygen. The increased unloading at the tissue level is called the Bohr effect.
The manner in which hemoglobin combines with oxygen is not quite clear, though much is known of the structure of hemoglobin. It contains 4 heme units, which contain iron and attach to a polypeptide. Each unit has a molecular weight of 17,000 and combine with one oxygen molecule. The iron of the heme is ferrous iron but it does not become ferric iron when oxygen is present. The combination is one of association; no electrons are transferred.
Plasma also carries oxygen, though not nearly so much. As noted in Part 1, a liter of plasma contains only 3. 0 ml of oxygen at a partial pressure of 100 mm Hg compared with the 200 ml carried by a liter of normal blood (which contains 12 g of hemoglobin). When hemoglobin is low or absent, the ability of the plasma to dissolve oxygen can be used to advantage. In fact, a resting man without any red cells at all can be sustained by exposing him to a partial pressure of 5000 mm Hg of oxygen.
In the normal person exposed to normal oxygen partial pressure, about 1% of this gas is carried by the plasma. We shall confine ourselves here to the delivery of oxygen by the oxygen-hemoglobin complex. Figure 275 will be required for this analysis.
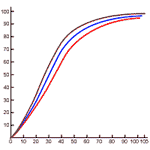 |
In the lungs, the association between oxygen and hemoglobin is determined by the partial pressure of oxygen, the partial pressure of carbon dioxide, and the temperature. These values are respectively 109 mm Hg, 38 mm Hg and 37 oC. In an average tissue, the partial pressure of O2 might be 40 mm Hg, the partial pressure of CO2 80 mm Hg, and the temperature 39 oC. The differences in temperature and partial pressure of CO account for the differences between the blue and red association curves of Figure 275.
The figure shows that at 40 mm Hg partial pressure of oxygen in the lungs, the blood contains 140 ml of oxygen per liter. If the situation in the tissues were the same, the blood would still contain 180 ml; only 55 ml of O2 would be supplied to the tissues per liter of blood.
But the tissues are dominated by the red association curve. They contain more carbon dioxide than the lungs and are4 in general hotter (except the skin). Both factors contribute to the dissociation of the hemoglobin--oxygen complex. In the prevailing conditions, the hemoglobin-oxygen complex contains 120 ml per liter of blood, so the carrying capacity, 190 ml O2 per liter, is converted into a delivery capacity of 70 ml per liter.
It is probable that very active tissues, especially muscle, can do even better. This leaves a large, untapped reserve for activity. Two organs which ordinarily excel at oxygen removal are the heart and liver. The heart depends entirely on aerobic metabolism and has a rather small reserve, while the liver and the organs which drain into it can obtain their energy needs anaerobically for rather long times; their aerobic reserve is quite small while their anaerobic reserve is very large.
The table which follows gives the values for the differences in oxygen concentration of arterial and venous blood as well as the corresponding oxygen utilization of the major organs at rest:
| Blood Flow (L / min) |
Arterio-Venous O2
difference (ml / L) |
O2
consumption (ml / min) |
|
| Brain | 0.7 | 50 | 35 |
| Heart | 0.3 | 100 | 30 |
| Liver and draining organs | 1.6 | 80 | 128 |
| Kindeys | 1.2 | 20 | 24 |
| Skin | 0.4 | 10 | 4 |
| Other: lungs, bones, glands, muscles | 1.8 | 17 | 30 |
| Total | 6.0 | 251 | |
This table may contain some surprising information. More than half the oxygen used by the body at rest is used by the liver and the gastro-intestinal tract (the spleen makes a very minor contribution). The remainder, consisting mostly of muscle and bone, uses very little oxygen, though it has a large blood flow. The kidneys, with a blood flow of 20% of the cardiac output consume less oxygen than the heart (5% of the cardiac output), or the brain (12% of the cardiac output). The skin, the largest organ in the body with about 20% of body weight, consumes virtually no blood and almost no oxygen.
A comparable table for an exercise like fast walking (3. 7 mph) follows:
| Blood Flow (L / min) |
Arterio-Venous O2
difference (ml / L) |
O2
consumption (ml / min) |
|
| Brain | 0.7 | 50 | 35 |
| Heart | 0.6 | 100 | 60 |
| Liver and draining organs | 1.6 | 80 | 128 |
| Kindeys | 1.2 | 20 | 24 |
| Skin | 0.5 | 26 | 13 |
| Other: lungs, bones, glands, muscles | 5.4 | 100 | 540 |
| Total | 10.0 | 800 | |
Note that the cardiac output is not quite doubled but oxygen utilization is more than tripled by this exercise. It should be noted further that the brain, liver and kidneys do not suffer from lack of blood or oxygen during exercise. Some information indicates that these organs actually have increased blood flow in exercise. The major changes which occur are in the skin (which becomes warmer because of the heat generated in exercise, so that both vasodilation and increased arterio-venous differences develop) and the remainder, most of which is skeletal muscle. The blood flow increases three-fold (see Chapter 12) and the oxygen extraction increases almost six-fold due to a combination of lowered oxygen tension and the altered hemoglobin-oxygen association curve in the active tissues. Oxygen utilization here increases eighteen fold.
The ability to deliver oxygen to tissues depends on:
These factors, which have been mentioned before, are all subject to disturbances which will be considered in Part 5.
4. Exchanges of Carbon Dioxide:
Carbon dioxide is carried from the tissues to the lungs by three different mechanisms. A small quantity, about 5%, is carried in physical solution. A larger quantity is carried by hemoglobin itself, 30%, but the greatest amount, 65%, is carried within the red cell by a complicated system composed as follows.
This point, which is of little quantitative significance in normal physiology, is emphasized to illustrate that in cases of defective hemoglobin or red cell formation, carbon dioxide transportation is not the main problem. Though little of it is carried in the plasma, all of it could be, provided the cardiac output were a little larger than usual.
(2) Combination with hemoglobin: Carbon dioxide diffuses across cell boundaries very easily; the erythrocyte is no exception. Once inside the erythrocyte it forms a loose complex with hemoglobin, something like the oxygen-hemoglobin complex. The compound so formed is called carbaminohemoglobin. This compound shows very strange and useful behavior which is strikingly analagous to the behavior or the oxygen-hemoglobin complex in the presence of carbon dioxide. Just as carbon dioxide reduces the ability of hemoglobin to hold oxygen, oxygen reduces the ability of the hemoglobin to hold carbon dioxide. Thus in the tissues, where the partial pressure of oxygen is low, carbaminohemoglobin is formed in substantial quantities. In the lungs, the high partial pressure of oxygen reduces the amount of carbaminohemoglobin which can exist and carbon dioxide must be released. About 30% of the carbon dioxide of blood is present as carbaminohemoglobin. This value refers to carriage, not delivery. The value for unloading varies with the circumstances, but it probably is only a small fraction of the total carbon dioxide moved from tissues to lung.
(3) Most carbon dioxide is carried in chemical combination as bicarbonate ion. This is formed in the tissues and is unloaded in the lungs.
Hemoglobin within the red cell is ordinarily combined with potassium, but in the absence of oxygen it has a greater affinity for the hydrogen ion than the potassium ion. Carbon dioxide formed in tissues (where oxygen is low) diffuses into the red cell. Here it forms carbonic acid by combination with water. This process, which is ordinarily a slow one is accelerated by an enzyme called carbonic anhydrase. Carbonic acid contains a readily available hydrogen ion and a bicarbonate ion. The hydrogen ion replaces the potassium ion previously associated with the hemoglobin; the potassium is "covered" by the bicarbonate which is left.
In the presence of oxygen, the affinity of hemoglobin for hydrogen ions is very much reduced, while its affinity for potassium is increased. Hydrogen ions released from hemoglobin react with bicarbonate ions released from potassium, and carbonic acid is formed. Carbonic acid so formed breaks down into carbon dioxide and water; the reaction rate is increased by carbonic arhydrase. (The student may wonder how it can be that carbonic anhydrase increases both the rate of formation and the rate of breakdown of carbonic acid. This property is quite characteristic of all enzymes: they increase reaction rates in both directions. Equilibrium comes about sooner in the presence of an enzyme, but the equilibrium is the same.
The effect of these processes is that erythrocyte potassium, mostly available to combine with the bicarbonate formed from carbonic acid in tissues, is mostly unavailable in the lungs, so that carbonic acid and hence carbon dioxide are released in the lung. The importance of this process may be judged by the fact that 65% of blood carbon dioxide is carried in this form.
The above reactions are summarized in the following chemical equations:
| TISSUES |
CO2 +
H2O <--> H2CO3
H2CO3 + KHb <--> HHb + KHCO3 |
| LUNGS |
HHb + O2 <--> HHbO2 HHbO2 + KHCO3 <--> H2CO3 + KHbO2 H2CO3 <--> H2O + CO2
|
The enzyme carbonic anhydrase is fairly widespread through the body. High concentrations are found in the kidney, the stomach, and the erythrocytes.
Through a combination of these
means, carbon dioxide is transported very readily from the cells to the tissues.
Quite often, oxygen transportation mechanisms can be defective, but carbon
dioxide is still moved easily. The body seems better designed to eliminate
carbon dioxide than to take in oxygen. This will be considered further in Chapter 19.
5. Disorders in the Movement of the Respiratory
Gases:
This section will be concerned
primarily with disorders in the movement of oxygen. These are sometimes
associated with abnormalities in carbon dioxide transfer, but, as has just been
indicated, carbon dioxide is eliminated from the body more effectively than
oxygen is taken in.
Altitude: At high
altitudes, the atmospheric pressure is lower than at sea level. The mole
fraction of oxygen, however, remains the same. Thus, the partial pressure of
oxygen in the alveolar air is reduced at high altitudes. This is ordinarily
tolerated with little or no difficulties at altitudes up to a mile. As the
altitude increases, more and more people suffer distress, particularly when they
attempt physical exertion. At the altitude of Mexico City (1.4 miles) persons
who are not natives experience so-called "mountain sickness" for a while. The
symptoms include labored breathing, headache, insomnia and increased heart rate,
even at rest. Within 3 to 10 days, one usually adjusts to the altitude, but it
is not clear that peak physical performance is possible. The 1969 Olympics may provide a test of this.
At 15,000 feet, adjustments are
still possible, and natives of the Andes and Himalayas are capable of perfoming
heavy labor at this altitude. These people show increased lung volume,
hemoglobin count, and tissue vascularization. Preliminary
studies indicate that these adjustments occur at least in part through natural
selection, and it may be that persons acclimatized to high altitude have
somewhat different body chemistry than those who live at sea level.
Altitudes higher than 18,000 feet
are not tolerated by man unless he breathes pure oxygen. Altitudes above 50,000
feet cannot be tolerated in any circumstances since so large a mole fraction of
the alveolar air is present as water vapor.
Artificial respiration:
The muscles which achieve respiration are controlled through the respiratory
centers of the medulla. These are in turn under the control of blood gases and
usually match respiratory efforts exactly to respiratory requirements. Certain
circumstances exist, however, in which the centers fail or the respiratory
muscles are paralyzed. One of the most dramatic of these is observed in
poliomyelitis which affects the medullary motor neurons of respiration.
Another, usually deliberately induced, occurs when large doses of curane are
given, for example, as in surgery. In both cases, recovery will occur if gas
exchanges are accomplished.
There are a number of methods for
producing such exchanges. Some involve the intermittent application of manual
pressure to the chest, but these have, to a large extent, been abandoned because
they are not very effective. These have been replaced by
"mouth-to-nose" methods, in which, after clearing the airway, one uses one's own
respiratory muscles to achieve gas exchanges in the patient.
In anesthetized patients who have
received curane to achieve the muscular relaxation essential for abdominal
surgery, a tight fitting tube is inserted into the trachea. If respiration
fails, this tube can be intermittently connected to a tank of gas which inflates
the lungs to the desired degree at the desired frequency. Deflation, in the
normal person, is a passive act, brought about by the elastic recoil of the
lungs and the associated structures. This type of artificial respiration is
called positive pressure respiration. It should not be attempted by
untrained persons, since there is a small but real danger that overzealous
inflation may result in explosion of the lung. Less serious degrees of
hyperinflation may result in hyperventilation, which may disable the
respiratory apparatus completely.
Neither of the above methods is
suitable for patients with poliomyelitis involving the motor neurons concerned
with respiration. Such persons must spend long periods--sometimes their entire
lives--in mechanical respirators, iron lungs. These operate on a fairly simple
principle. The body is placed in a metal cylinder, which is sealed around the
neck by a rubber diaphragm. A pump cycles air into and out of the cylinder. As
air is forced into the cylinder, the body is compressed and the lungs empty. The
withdrawal of air, lowering the pressure outside the body causes inspiration.
The arrangement is shown in Figure 277. Respiration produced in
this manner is said to be positive-and-negative-pressure respiration.
No method of artificial
respiration can be successful if the airway is blocked. If irritation due to a
foreign object touching the vocal cords results in laryngospasm, the
airway must be opened before any attempt is made at artificial respiration.
Sometimes, this is best done by tracheotomy, opening the trachea below
the larynx. The resemblance between this procedure and a simple criminal cutting
of the throat is so great that uninformed bystanders are often confused, and
sometimes the well-meaning person attempting an emergency tracheotomy is treated
with the considerable barbarity, to his detriment and that of the patient.
Another limitation of artificial
respiration is not always recognized by the person administering it. In most
cases, where respiration has ceased, the heart has also ceased its beat.
Adequate ventilation of the lungs, oxygenating the blood in the lungs, does no
good at all since that blood is not going anywhere. A pulseless person who is
not breathing (which often happens in persons who have had high voltage
electrical shocks) requires measures to restore his circulation first.
Artificial respiration may not be necessary after circulation is restored. If
both the circulatory and respiratory functions are arrested, hospital treatment
is indicated. Unfortunately, damage to vital organs such as the brain begins
within 5 minutes and it is rarely the case that a non-hospitalized patient can
be brought to a properly equipped emergency room soon enough to do benefit.
There is a
poorly understood phenomenon which sometimes makes artificial respiration seem
useful even in circulatory arrest. A sudden mechanical stimulus applied to the
chest just over the heart may restore a normal heart beat. The artificial
respiration probably produces this effect only incidentally, and it is probably
better to attempt to restore circulation by a few hard punches over the heart.
Artificial respiration can begin when (and if) the heart beat is restored. The
whole procedure should, however, be used only to support the patient till he can
be brought to a hospital.
Pulmonary edema: Some of
the causes of pulmonary edema have been considered in Chapters 15, 16,
and 17. Whether pulmonary edema results from
left heart failure, mitral stenosis, or abnormal permeability of the pulmonary
capillaries to proteins, the effect is always to interpose an additional fluid
layer between the alveolar air sacs and the pulmonary capillaries. The increased
distance through which oxygen must diffuse results in defective oxygenation of
the lung even though alveolar oxygen is normal. The difficulty can sometimes be
remedied by the administration of pure oxygen, and so the greater diffusion
distance is compensated by the greater partial pressure of oxygen in the
alveoli.
Emphysema and tuberculosis
destroy pulmonary tissue in different ways, both have the effect of reducing
oxygenation of the blood. Emphysema has the further effect that because alveolar
ventilation is poor the partial pressure of carbon dioxide in the alveolar air
sacs rises. At first this produces discomfort and increased alveolar
ventilation. In time, the victim of emphysema adjusts to a higher partial
pressure of carbon dioxide in his alveolar air sacs, and hence in his arterial
blood, than is normally tolerable. These diseases, mentioned briefly in Chapter 17, are discussed in detail in textbooks of
medicine.
Anemia has been discussed
in Chapter 9. The consequences of most
anemias are that the oxygen and carbon dioxide carrying capacity of the blood
are diminished per unit volume of blood. To a certain extent, the diminution in
carrying capacity per unit volume is mitigated by an increased flow of blood.
Anemic blood is less viscous than normal and the cardiac output of the anemic
person is therefore higher than that of the normal.
Carbon monoxide, which
makes a fairly firm bond with hemoglobin, reduces the oxygen carrying capacity
of the blood. Unlike anemia, carbon monoxide poisoning does not change the
physical properties of blood. Thus the flow of blood remains normal and the
reduced oxygen carrying capacity is uncompensated. The carbon
monoxide-hemoglobin complex will break down in time, particularly if the partial
pressure oxygen is made very high, but the process is slow and irreversible
damage to the brain may come about. Many cases of carbon monoxide poisoning are
deliberate suicide attempts; others result from a faulty combustion of gas or
organic fuels in indoor heating devices. Carbon monoxide poisoning is always to
be feared in firefighting, since the carbon of wood, when burning rapidly, does
not oxidize completely.
Shock, discussed in Chapter 16, results in lowered cardiac output. The
oxygen carrying capacity of each liter of blood may be quite normal. Yet
oxygenation of the tissues may be defective because so little blood comes to
them per unit time. The treatment of shock is directed to the collapsing
circulation rather than the adequate respiratory system. Hyperbaric oxygen,
which increases the oxygen content of blood, may be of some value in shock
treatment, but it should not be used until proper steps have been taken to
correct the circulatory defect.
Histotoxic anoxia: The
delivery of oxygen from lungs to cells requires that the cells be able to
dislodge it. Sometimes they are unable to do so. This occurs very dramatically
in cyanide poisoning; the entire respiratory process may be quite normal, but
the poisoned cells are unable to "unload" the oxygen from oxyhemoglobin.
Since carbon dioxide is necessary
to accomplish dissociation of the oxygen-hemoglobin complex, and since it can be
lowered in the tissues by hyperventilation, a rather paradoxical situation may
develop in which hyperventilation leads to faulty transport of oxygen. In many
cases, hyperventilation is involuntary and unconscious. Such hyperventilating
patients may have symptoms referrable to oxygen deficiency in various organs.
This will be discussed again in the next chapter.
Continue to Chapter 19.