

 Mimicking Bone Imagine a sponge-like metal foam, a porous material that comprises or coats a medical implant. Now assume you can create that material using tantalum, one of the most biocompatible metals known to man, and that you can tailor the tantalum material to match the mechanical aspects and load-bearing functions of real bone. This new material, called Trabecular Metal, has been developed by the Implex Corporation and is being studied in collaboration with the University of Notre Dame, Zimmer, Inc., and Implex Corp. The cellular structure of Trabecular Metal resembles that of trabecular bone, the type of bone found in the joints of the body and replaced by joint implants. One of the unique characteristics of Trabecular Metal is that it approximates the physical and mechanical properties of bone more closely than other prosthetic materials. Its high strength-to-weight ratio and low elasticity results in nearly physiological loading, which encourages living bone to grow into the porous structure of Trabecular Metal more quickly and form a stronger bond than with other synthetic porous materials. The bond formed between the implant and living tissue extends the life of an implant, avoiding the expensive and painful revision surgeries often required about 20 years following an implantation which used traditional cement fixation techniques. Although Trabecular Metal offers great promise as a material for joint implants, machining processes for this material are not mature. To date no products have been manufactured which required bending Trabecular Metal, even though this less expensive process would simplify fabrication. This is one of the projects that Steven R. Schmid, associate professor of aerospace and mechanical engineering, and Glen L. Niebur, assistant professor of aerospace and mechanical engineering, along with Paul S. Nebosky, a graduate student in the department, are researching. They, in partnership with Implex and Zimmer, are developing manufacturing processes that are more efficient, while also designing the materials and tooling that allow greater deformation of Trabecular Metal without damaging the porous structure that is critical to bone ingrowth properties. The goal of the Notre Dame group is to form implants from the material while preserving optimum throughput in the foam-producing reactors. One approach is to bend flat pieces into a variety of shapes, such as the bone ingrowth pads on a hip implant. In recent experiments Schmid, Niebur, and Nebosky have achieved over 90-degree bends in specimens roughly two millimeters in thickness, a significant accomplishment for a material that does not normally deform to this high of a degree. Using different mathematical models of the foam behavior, Notre Dame researchers are also attempting to predict the viability of manufacturing operations before designing and building expensive tooling.   Trabecular Metal, a material composed of tantalum on a graphite scaffold, acts and looks like trabecular bone, bottom photo, the type of bone that supports the joints of the body. The porosity and felixibility of Trabecular Metal encourgae bone to grow into its pores. Bonds between this material and ligament tissue are almost as strong as the bonds between the tissue and real bone. |
Biomaterials The mechanical devices implanted into biological systems are important; they have increased the length and quality of life and promise to provide even greater benefits in the near future. Equally as important are the materials from which they are made, as well as the materials that “glue” them into place. These “biomaterials” include living tissue as well as synthetic substances. As they continue to pursue minimally invasive procedures and other avenues of research, Notre Dame engineers, biologists, and chemists are working together to improve existing materials and create new ones that will last longer, be more wear resistant, and even strengthen bone.  Understanding materials and how to engineer their properties on the molecular
level is vital. Why? “You can’t solve this problem if you
don‘t understand the molecules,” says Edward
J. Maginn, associate
professor of chemical engineering, of the cartilage wear project he is
working on with Timothy C. Ovaert, professor of aerospace and mechanical
engineering, and Schmid. “The phenomenon -- that the proteins naturally
present in synovial fluid lubricate cartilage joints much better than
polyethylene joints -- is due to molecular-level processes. Using molecular
modeling, we can simulate proteins in the synovial fluid at the atomistic
level as they interact with both natural cartilage and synthetic material
to understand how they lubricate surface areas. With this information
we can then work together on the macroscopic problem, engineering better,
more wear-resistant materials.” Understanding materials and how to engineer their properties on the molecular
level is vital. Why? “You can’t solve this problem if you
don‘t understand the molecules,” says Edward
J. Maginn, associate
professor of chemical engineering, of the cartilage wear project he is
working on with Timothy C. Ovaert, professor of aerospace and mechanical
engineering, and Schmid. “The phenomenon -- that the proteins naturally
present in synovial fluid lubricate cartilage joints much better than
polyethylene joints -- is due to molecular-level processes. Using molecular
modeling, we can simulate proteins in the synovial fluid at the atomistic
level as they interact with both natural cartilage and synthetic material
to understand how they lubricate surface areas. With this information
we can then work together on the macroscopic problem, engineering better,
more wear-resistant materials.”Biomaterials, natural or synthetic, must also be in tune with the body’s many systems. As strong as the body can be, it is delicately balanced. Not every metal or composite is appropriate for use as a biomaterial. For example, while steel and aluminum are common engineering materials, they should not be implanted into biological matter. Tantalum is the most biocompatible metal known. High-density polyethylene is also highly biocompatible; it is basically the same polymer used in milk jugs. And, polyurethane has been used extensively in artificial heart valves. According to Ovaert, polymers are ideal for biomedical applications because there is a low level of toxicity with the body. “The human body accepts polymeric materials much more readily than a lot of metals or other types of inorganic materials,” he says, “and they’re inexpensive to process. Plastics (polymers) also offer greater wear-resistance features in some applications.” Mason, Schmid, and Davide A. Hill, associate professor of chemical engineering, are working to develop new polymers for use in hip replacement and spinal fixation applications. “The requirements for strength, fluidity, and viscosity for the material in the hip application are completely different from a spinal fixation project,” says Mason. “For the hip application, we are working to modify bone cement.” Traditional cements are currently used in thin layers between an implant and biological tissue, but new applications -- like the minimally invasive procedures the Notre Dame group is developing -- stretch the limits of the cements’ properties.  One of the main issues under consideration is thermal necrosis, the term
for the damage caused by the heat generated when a polymer cures in the
body. If the temperature is too high, the tissue surrounding the cement
is destroyed. “Bone cement heats when it hardens, and we’ve
been working to modify that, bringing the temperature down to more acceptable
levels,” says Hill. “We’ve also been studying photocurable
polymers, materials similar to what a dentist puts into a cavity to seal
it. We know that photocurable polymers heat very little when they cure,
which means less damage to surrounding tissue. What we are exploring
is whether or not we can design a new material, a biocompatible photocurable
polymer.” One of the main issues under consideration is thermal necrosis, the term
for the damage caused by the heat generated when a polymer cures in the
body. If the temperature is too high, the tissue surrounding the cement
is destroyed. “Bone cement heats when it hardens, and we’ve
been working to modify that, bringing the temperature down to more acceptable
levels,” says Hill. “We’ve also been studying photocurable
polymers, materials similar to what a dentist puts into a cavity to seal
it. We know that photocurable polymers heat very little when they cure,
which means less damage to surrounding tissue. What we are exploring
is whether or not we can design a new material, a biocompatible photocurable
polymer.”Creating new materials is also the thrust of Ryan K. Roeder’s research. Roeder, an assistant professor of aerospace and mechanical engineering, is working to design biomaterials that more closely match the mechanical properties of human bone. “Bone is, in my opinion, the most original and most incredible composite material around,” says Roeder. “While there’s a great deal of excitement surrounding tissue engineering today, we still don’t have a synthetic material that mechanically functions identically to bone.”  Most of the materials currently used to replace or strengthen bone are
an order of magnitude stiffer than bone. What often happens, according
to Roeder, is that the stiffer material carries the load instead of the
bone carrying the load. It’s called stress shielding, and when
it occurs, the bone -- signaled by a decreased load and, hence, a decreased
need to be strong -- starts to resorb and pull away from the implant.
An additional parameter of Roeder’s ideal bone substitute is that
it should be able to serve as a vehicle for delivering growth factors
or cells to help bone repair and strengthen itself. Most of the materials currently used to replace or strengthen bone are
an order of magnitude stiffer than bone. What often happens, according
to Roeder, is that the stiffer material carries the load instead of the
bone carrying the load. It’s called stress shielding, and when
it occurs, the bone -- signaled by a decreased load and, hence, a decreased
need to be strong -- starts to resorb and pull away from the implant.
An additional parameter of Roeder’s ideal bone substitute is that
it should be able to serve as a vehicle for delivering growth factors
or cells to help bone repair and strengthen itself. Given the nature of bone, this is a tall order. Bone consists of several hierarchical structural features, which at the simplest level are comprised of a biopolymer, namely collagen. The collagen is reinforced with elongated ceramic particles or bone mineral. The particles are calcium phosphates with a complex composition and crystal structure, which is most closely resembled by hydroxyapatite, the material Roeder grows in aqueous solutions in his lab and expects to use as a biomimetic reinforcement phase in new synthetic biocomposites. Roeder anticipates that, as the field of biomaterials develops, there will be a shift from using relatively few materials for many different devices and applications to designing devices using a variety of unique materials, whose properties -- mechanical, biological, or functional -- have been tailored for a specific application. |
|
|
|
 Drawing a Bead on Disease Between growing photosynthetic bacteria that could eventually be used as the primary ingredient of a mobile septic system for space travel and using the proteins in red blood cells to identify other deficient cells in the body, Agnes E. Ostafin, assistant professor of chemical engineering, is developing nanoshell biosensors for use in early disease-detection systems. These nanometer sized inorganic beads can be “programmed” to seek out specific proteins in the body. Because they are hollow, they can be loaded with dyes and other materials that can be detected by light, X-rays, or electrons. “The idea,” says Ostafin, “is to be able to detect features of tumor cells and use these features to track the progress of the tumor, the effectiveness of the therapy, or to design targeted drugs.” Currently, she is working with the Bayer Corporation, which is interested in using the beads in urine analysis and in developing the nanoshells for use in in vitro applications. While the technology could obviously be useful to identify diseases in their early stages, it also offers a new method of drug delivery. For example, porous nanoshells could be filled with insulin and placed on a “patch” below the skin, a patch designed to release insulin when excess glucose is detected.  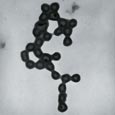 Silicate is one of the materials used to form the nanoshells. Ostafin’s group has also successfully created calcium phosphate nanoshells. Hydroxyapatite, a form of calcium phosphate, is the material found in bone. If filled with a hormone that stimulates bone growth, the calcium phosphate nanoshells could provide an effective treatment for osteoporosis and bone replacement therapy. However, Ostafin cautions that one nanoshell does not fit all applications. Each shell is designed differently for each specific application. For more information on the nanoshell projects, visit the Nano-scale Bioengineering Laboratory web site at http://www.nd.edu/ ~aostafin. |
 On any given day an average of 34,000 units of red blood cells are
needed ... for trauma victims, heart surgeries, organ transplants,
and patients receiving treatment for diseases such as leukemia,
sickle cell anemia, and thalassemia. Each unit of whole blood is
normally separated into several components. Red blood cells, which
carry oxygen and are used to treat anemia, can be stored under
refrigeration at 4 degrees Celsius for approximately 40 days. They
may also be frozen for up to 10 years. Platelets, vital in controlling
bleeding and often used in patients with leukemia and other forms
of cancer, can be stored at room temperature for up to five days.
Fresh frozen plasma, also used to control bleeding, is usually
kept in a frozen state and is viable for up to one year. Containing
a few specific clotting factors, cryoprecipitated AHF is made from
fresh frozen plasma and can be frozen for up to a year. Granulocytes,
sometimes used to fight infections, must be transfused within 24
hours of donation.
On any given day an average of 34,000 units of red blood cells are
needed ... for trauma victims, heart surgeries, organ transplants,
and patients receiving treatment for diseases such as leukemia,
sickle cell anemia, and thalassemia. Each unit of whole blood is
normally separated into several components. Red blood cells, which
carry oxygen and are used to treat anemia, can be stored under
refrigeration at 4 degrees Celsius for approximately 40 days. They
may also be frozen for up to 10 years. Platelets, vital in controlling
bleeding and often used in patients with leukemia and other forms
of cancer, can be stored at room temperature for up to five days.
Fresh frozen plasma, also used to control bleeding, is usually
kept in a frozen state and is viable for up to one year. Containing
a few specific clotting factors, cryoprecipitated AHF is made from
fresh frozen plasma and can be frozen for up to a year. Granulocytes,
sometimes used to fight infections, must be transfused within 24
hours of donation.