Synthetic Secrets
Researchers in our group have formulated simple chemical routes for preparing nanomaterials for use in a wide variety of fields. The links below aim to provide detailed step-by-step procedures, along with publications where the method was used, for synthesizing and fabricating many of these materials and devices.
Please cite the proper sources for all methods used from this page and, as always, follow standard laboratory safety practices.
- IPCE Derivation Derivation of the equation used for calculating Incident Photon to Carrier Efficiency (IPCE)
- Making QDSCs - Video Detailed video showing the step-by-step process of making high efficiency quantum dot sensitized solar cells. References:
- Synthesizing Fluorescent Ag8 Clusters
Synthesis of fluorescent Ag8 nanoclusters surrounding a Ag nanoparticle shell
References:
430. Realizing Visible Photoactivity of Metal Nanoparticles: Excited-State Behavior and Electron-Transfer Properties of Silver (Ag8) Clusters. Chen, W. T.; Hsu, Y. J.; Kamat, P. V. J. Phys. Chem. Lett. 2012, 3, 2493–2499.
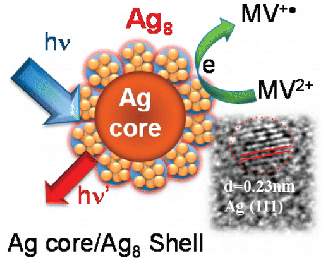 Silver nanoclusters complexed with dihydrolipoic acid (DHLA) exhibit molecular-like excited-state properties with well-defined absorption and emission features. The 1.8 nm diameter Ag nanoparticles capped with Ag8 clusters exhibit fluorescence maximum at 660 nm with a quantum yield of 0.07%. Although the excited state is relatively short-lived (τ 130 ps), it exhibits significant photochemical reactivity. By introducing MV2+ as a probe, we have succeeded in elucidating the interfacial electron transfer dynamics of Ag nanoclusters. The formation of MV+• as the electron-transfer product with a rate constant of 2.74 × 1010 s–1 confirms the ability of these metal clusters to participate in the photocatalytic reduction process. Basic understanding of excited-state processes in fluorescent metal clusters paves the way toward the development of biological probes, sensors, and catalysts in energy conversion devices.
Silver nanoclusters complexed with dihydrolipoic acid (DHLA) exhibit molecular-like excited-state properties with well-defined absorption and emission features. The 1.8 nm diameter Ag nanoparticles capped with Ag8 clusters exhibit fluorescence maximum at 660 nm with a quantum yield of 0.07%. Although the excited state is relatively short-lived (τ 130 ps), it exhibits significant photochemical reactivity. By introducing MV2+ as a probe, we have succeeded in elucidating the interfacial electron transfer dynamics of Ag nanoclusters. The formation of MV+• as the electron-transfer product with a rate constant of 2.74 × 1010 s–1 confirms the ability of these metal clusters to participate in the photocatalytic reduction process. Basic understanding of excited-state processes in fluorescent metal clusters paves the way toward the development of biological probes, sensors, and catalysts in energy conversion devices. - Sensitizing TiO2 with CQDs
Attaching TOPO capped CdSe quantum dots to TiO2 films using with/without linkers
References:
408. Tracking the Adsorption and Electron Injection Rates of CdSe Quantum Dots on TiO2: Linked Versus Direct Attachment Pernik, D.; Tvrdy, K.; Radich, J. G.; Kamat, P. V. J. Phys. Chem. C 2011, 133 (24), pp 9607–9615.
- Etching TiO2 Nanotubes
Anodic etching of Ti foil substrate to give TiO2 nanotubes
References:
386. Got TiO2 Nanotubes? Lithium Ion Intercalation can Boost Their Photoelectrochemical Performance Three-Fold Meekins, B. H.; Kamat, P. V. ACS Nano 2009, 3, 3437–3446. NDRL 4821
382. Disassembly, Reassembly and Photoelectrochemistry of Etched TiO2 Nanotubes Baker, D. R.; Kamat, P. V. J. Phys. Chem. C 2009, 113, 17967-17972. NDRL 4816
373. Photosensitization of TiO2 Nanostructures with CdS Quantum Dots. Particulate versus Tubular Support Architectures Baker, D. R.; Kamat, P. V. Adv. Funct. Mater. 2009, 19, 805-811. NDRL 4771
- Semiconductor Colloids
- Colloidal Metals
- Nanorods
- Core Shell Particles
- Nanostructured Semiconductor Films
- EPD of Molecular Cluster Films
- EPD of Single Wall Carbon Nanotubes
- EPD of CdSe films
420. Mn-Doped Quantum Dot Sensitized Solar Cells. A Strategy to Boost Efficiency over 5% Santra, P. K.; Kamat, P. V. J. Am. Chem. Soc. 2012, 134 (5), 2508–2511.
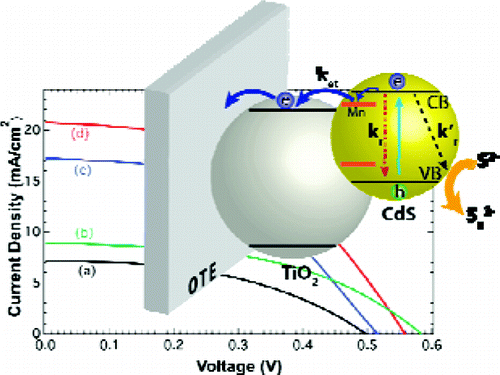 To make Quantum Dot Sensitized Solar Cells (QDSC) competitive, it is necessary to achieve power conversion efficiencies comparable to other emerging solar cell technologies. By employing Mn2+ doping of CdS, we have now succeeded in significantly improving QDSC performance. QDSC constructed with Mn-doped-CdS/CdSe deposited on mesoscopic TiO2 film as photoanode, Cu2S/Graphene Oxide composite electrode, and sulfide/polysulfide electrolyte deliver power conversion efficiency of 5.4%.
To make Quantum Dot Sensitized Solar Cells (QDSC) competitive, it is necessary to achieve power conversion efficiencies comparable to other emerging solar cell technologies. By employing Mn2+ doping of CdS, we have now succeeded in significantly improving QDSC performance. QDSC constructed with Mn-doped-CdS/CdSe deposited on mesoscopic TiO2 film as photoanode, Cu2S/Graphene Oxide composite electrode, and sulfide/polysulfide electrolyte deliver power conversion efficiency of 5.4%.
416. Cu2S-Reduced Graphene Oxide Composite for High Efficiency Quantum Dot Solar Cells . Overcoming the Redox Limitations of S2-/Sn2- at the Counter Electrode Radich, J. G.; Dwyer, R.; Kamat, P. V. J. Phys. Chem. Lett. 2011, 2, 2453–2460.
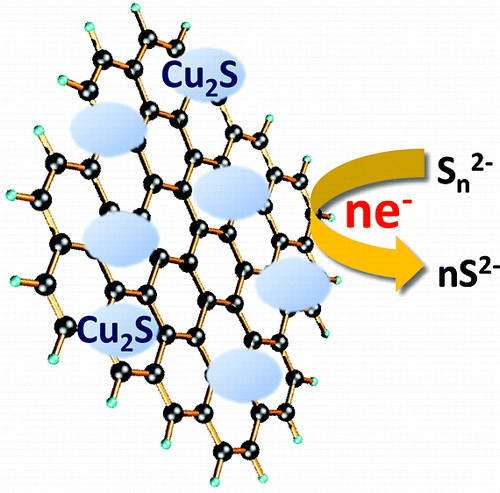 Polysulfide electrolyte that is employed as a redox electrolyte in quantum dot sensitized solar cells provides stability to the cadmium chalcogenide photoanode but introduces significant redox limitations at the counter electrode through undesirable surface reactions. By designing reduced graphene oxide (RGO)-Cu2S composite, we have now succeeded in shuttling electrons through the RGO sheets and polysulfide-active Cu2S more efficiently than Pt electrode, improving the fill factor by 75%. The composite material characterized and optimized at different compositions indicates a Cu/RGO mass ratio of 4 provides the best electrochemical performance. A sandwich CdSe quantum dot sensitized solar cell constructed using the optimized RGO-Cu2S composite counter electrode exhibited an unsurpassed power conversion efficiency of 4.4%.
Polysulfide electrolyte that is employed as a redox electrolyte in quantum dot sensitized solar cells provides stability to the cadmium chalcogenide photoanode but introduces significant redox limitations at the counter electrode through undesirable surface reactions. By designing reduced graphene oxide (RGO)-Cu2S composite, we have now succeeded in shuttling electrons through the RGO sheets and polysulfide-active Cu2S more efficiently than Pt electrode, improving the fill factor by 75%. The composite material characterized and optimized at different compositions indicates a Cu/RGO mass ratio of 4 provides the best electrochemical performance. A sandwich CdSe quantum dot sensitized solar cell constructed using the optimized RGO-Cu2S composite counter electrode exhibited an unsurpassed power conversion efficiency of 4.4%.
