News From the Kamat Lab
Sense and Shoot
Read the latest paper from the Kamat Lab!
Sense and Shoot: Simultaneous Detection and Degradation of Low Level Contaminants using Graphene Based Smart Material Assembly
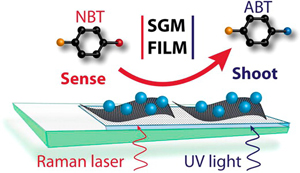
Abstract: Smart material nanoassemblies that can simultaneously sense and shoot low level contaminants from air and water are important for overcoming the threat of hazardous chemicals. Graphene oxide (GO) sheets deposited on mesoscopic TiO2 films that underpin the deposition of Ag nanoparticles with UV-irradiation provide the foundation for the design of a smart material. The Ag particle size is readily controlled through precursor concentration and UV irradiation time. These semiconductor – graphene oxide – metal (SGM) films are SERS active and hence capable of sensing aromatic contaminants such as nitrobenzenethiol (NBT) in nanomolar range. Increased local concentration of organic molecule achieved through interaction with 2D carbon support (GO) facilitates low-level detection of contaminants. Upon UV irradiation of the NBT loaded SGM film, one can induce photocatalytic transformations. Thus, each component of the SGM film plays a pivotal role in aiding the detection and degradation of a contaminant dispersed in aqueous solutions. The advantage of using SGM films as multipurpose "detect and destroy" systems for nitroaromatic molecule is discussed.
Langmuir - Cover Article

Congratulations to Jeff Christians on the cover art for the journal Langmuir! The cover art appeared in this month's issue of Langmuir to go along with Prashant, Jeff, and James's review article on hole transfer entitled, Quantum Dot Solar Cells: Hole Transfer as a Limiting Factor in Boosting the Photoconversion Efficiency.
About the Image: The artwork illustrates photoinduced charge-transfer processes in nanostructured solar cells. Top: Quantum dot solar cells (QDSCs). Center: Nanowire solar cells (NWSCs). Bottom: Extremely thin absorber (ETA) solar cells. Efficiencies greater than 5% have recently been achieved. The associated Invited Feature Article focuses on the limitations imposed by slow hole transfer and its role in the stability and power conversion efficiency of metal chalcogenide solar cells. Strategies to improve the rate of hole transfer through surface-modified redox relays offer new opportunities to overcome the hole-transfer limitation.
Switching the Reaction Course of CO2
Read the latest paper from the Kamat Lab!
Switching the Reaction Course of Electrochemical CO2 Reduction with Ionic Liquids
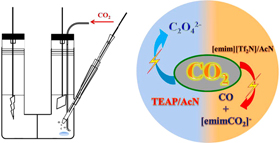
Abstract: The ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([emim][Tf2N]) offers new ways to modulate the electrochemical reduction of carbon dioxide. [emim][Tf2N], when present as the supporting electrolyte in acetonitrile, decreases the reduction overpotential at a Pb electrode by 0.18 V as compared to tetraethylammonium perchlorate as the supporting electrolyte. More interestingly, the ionic liquid shifts the reaction course during the electrochemical reduction of carbon dioxide by promoting the formation of carbon monoxide instead of oxalate anion. With increasing concentration of [emim][Tf2N], a carboxylate species with reduced CO2 covalently bonded to the imidazolium ring is formed along with carbon monoxide. The results highlight the catalytic effects of the medium in modulating the CO2 reduction products.
James to Auburn

Join us in congratulating Dr. James Radich as he will be starting a tenure track faculty position in the Department of Chemical Engineering at Auburn University. James has been a member of the Kamat group since he came to Notre Dame as a graduate student in 2009, receiving his Ph. D. in April, 2014, and will begin his new position at Auburn this June. As a faculty member, James will be seeking to develop a strong research program specializing in material science and electrochemistry. Congratulations James on this great accomplishment!
KamatLab Youtube Channel

Our group has published a variety of videos and webinar presentations through the website SlideShare. Because SlideShare is no longer supporting "Slidecasts" (Power Point presentations with audio) we have begun migrating all of our webinar presentations over to Youtube. You can now follow us on Youtube and see all of our latest updates and presentations, just click the link below!
How Does a SILAR CdSe Film Grow?
Read the latest paper from the Kamat Lab!
How Does a SILAR CdSe Film Grow? Tuning the Deposition Steps to Suppress Interfacial Charge Recombination in Solar Cells
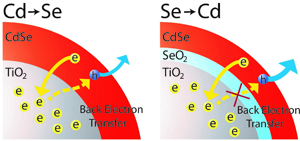
Abstract: Successive Ionic Layer Adsorption and Reaction (SILAR) is a popular method of depositing the metal chalcogenide semiconductor layer on the mesoscopic metal oxide films for designing quantum dot sensitized solar cell (QDSSC) or Extremely Thin Absorber (ETA) solar cells. While this deposition method exhibits higher loading of light absorbing semiconductor layer than direct adsorption of pre-synthesized colloidal quantum dots, the chemical identity of these nanostructures and evolution of interfacial structure are poorly understood. We have now analyzed step-by-step SILAR deposition of CdSe films on mesoscopic TiO2 nanoparticle films using x-ray absorption near-edge structure analysis and probed the interfacial structure of these films. The film characteristics interestingly show dependence on the order in which the Cd and Se are deposited, and the CdSe-TiO2 interface is affected only during the first few cycles of deposition. Development of SeO2 passivation layer in the SILAR-prepared films to form TiO2/SeO2/CdSe junction facilitates an increase in photocurrents and power conversion efficiencies of quantum dot solar cells when these films were integrated as photoanodes in a photoelectrochemical solar cell.
Webinar - Band Gap Tunining in CdSeS NWs

Watch this ACS LiveSlides presentation on the synthesis of CdSeS nanowires. This webinar describes the tuning of the composition of the NWs by the ratio of Se:S precursor. This allows for the modulation of the NW band gap between 2.36 eV and 1.79 eV. The presentation is based off of the paper, recently published in Journal of Physical Chemistry Letters, entitled:
CdSeS Nanowires. Compositionally Controlled Band Gap and Exciton Dynamics.Abstract: CdS, CdSe and ternary CdSexS(1-x) are some of the most widely studied II-VI semiconductors due to their wide range of applications and promising performance in numerous systems. One-dimensional semiconductor nanowires offer the ability to conduct charges efficiently along the length of the wire which has potential charge transport benefits compared to nanoparticles. Herein, we report a simple, inexpensive synthetic procedure for high quality CdSeS nanowires where the composition can be easily modulated from pure CdSe to pure CdS by simply adjusting the Se:S precursor ratio. This allows for tuning of the absorption and emission properties of the nanowires across the visible spectrum. The CdSeS nanowires have a wurtzite crystal structure and grow along the [001] direction. As measured by femtosecond transient absorption spectroscopy, the short component of the excited state lifetime remains relatively constant at ~10 ps with increasing Se; however the contribution of this short lifetime component increased dramatically from 8.4% to 57.7% with increasing Se content. These CdSeS nanowires offer facile synthesis and widely adjustable optical properties, characteristics which give them broad potential applications in the fields of optoelectronics, and photovoltaics.
Dr. James Radich

Congratulations to Dr. James Radic h on successful completion of his Ph. D. in Chemical and Biomolecular Engineering! James successfully defended his doctoral thesis entitled:
Reduced Graphene Oxide-Based Nanoassembiles for Energy Storage.
Congratulations on completion of your Ph. D. James!
Dr. Sachidananda Krishnamurthy

Congratulations to Dr. Sachi Krishnamurthy on successful completion of his Ph. D. in Chemistry! Sachi successfully defended his doctoral thesis entitled:
Graphene-Based Assemblies: Electron Transfer Processes and Energy Conversion Applications.
Congratulations on completion of your Ph. D. Sachi!
Editorial on Citation
Read this editorial, entitled "Cite with a Sight," from Prashant and Journal of Physical Chemistry Letters Editor-in-Chief George C. Schatz on the important area of citations in scientific research.
"Citing references in a scientific paper is becoming an increasingly important albeit challenging experience as the volume of scientific literature continues to explode. Despite the increasing average number of cited references in published papers, important and relevant papers are sometimes absent. Journal guidelines may recommend or even require the total number of citations be kept to a modest level. Consequently, authors need a balanced approach in identifying and including only the most relevant citations. In this Editorial, we discuss the importance of selecting and citing references correctly. We also discuss the impact of cited references on published research and their role in advancing the scientific discipline."
Hydrogen Evolution with Gold Clusters
Read the latest paper from the Kamat Lab!
Glutathione Capped Gold Nanoclusters as Photosensitizers. Visible Light Induced Hydrogen Generation in Neutral Water
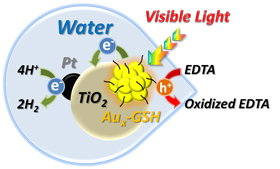
Abstract: Glutathione capped metal nanoclusters (Aux-GSH NCs) which exhibit molecular like properties are employed as a photosen-sitizer for hydrogen generation in a photoelectrochemical cell (PEC) and a photocatalytic slurry reactor. The reversible re-duction (E0= -0.63 V vs. RHE) and oxidation (E0= 0.97 V and 1.51 V vs. RHE) potentials of these metal nanoclusters make them suita-ble for driving the water splitting reaction. When a mesoscopic TiO2 film sensitized by Aux-GSH NCs was used as the pho-toanode with a Pt counter electrode in aqueous buffer solution (pH = 7), we observe significant photocurrent activity under visible light (400 - 500 nm) excitation. Additionally, sensitizing Pt/TiO2 nanoparticles with Aux-GSH NCs in an aqueous slurry system and irradiating with visible light produced H2 at a rate of 0.3 mmole of hydrogen/hr/gram of Aux-GSH NCs. The rate of H2 evolution was significantly enhanced (~5 times) when a sacrificial donor, such as EDTA, was introduced into the sys-tem. Using metal nanoclusters as a photosensitizer for hydrogen generation lays the foundation for the future exploration of other metal nanoclusters with well controlled numbers of metal atoms and capping ligands.
Size Dependent Electron Transfer from CdSe to GO
Read the latest paper from the Kamat Lab!
CdSe-Graphene Oxide Light Harvesting Assembly. Size Dependent Electron Transfer and Light Energy Conversion Aspects
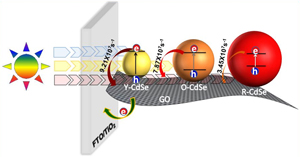
Abstract: Excited state interaction between CdSe quantum dots (QD) of different sizes (2.3, 3.2 and 4.2 nm diameter) and graphene oxide (GO) has been probed by depositing them as films on conducting glass electrodes. The emission of smaller CdSe QDs (2.3 nm) is quenched by GO three times faster than that of larger QDs (4.2 nm). Electrophoretic deposition method has allowed us to sequentially deposit single or multiple layers of different sized QDs and GO assemblies on conducting glass electrode and achieve the modulation of photoresponse in photoelectrochemical solar cells. Superior photoconversion efficiency through the incorporation of GO is attributed to the improved charge separation in the composite assembly.
JACS & JPC Lett. Virtual Issue - Perovskites
Prashant, with the help of The Journal of the American Chemical Society and The Journal of Physical Chemistry Letters, has put together a virtual issue on organometal perovskite photovoltaics. This issue highlights some of the important work in JACS and JPC Lett. on this exciting new field of photovoltaic research.
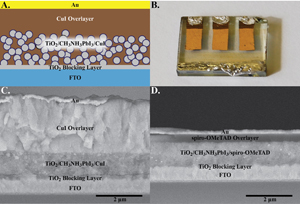
Read all of these great papers free of charge for a limited time! Including our recent paper where we use Copper Iodide as an inexpensive alternative hole conductor in perovskite solar cells.
An Inorganic Hole Conductor for Organo-Lead Halide Perovskite Solar Cells. Improved Hole Conductivity with Copper Iodide Christians, J. A.; Fung, R. C. A.; Kamat, P. V. J. Am. Chem. Soc. 2014, 136 (2), 758-764.
Also, read Prashant's editorial on this exciting new research field,
Organometal Halide Perovskites for Transformative Photovoltaics P.V. Kamat, J. Am. Chem. Soc. 2014, 136 (10), 3713–3714.
Feature Article - Hole Transfer in QDSCs
Read the latest paper from the Kamat Lab - a feature article in the journal Langmuir!
Quantum Dot Solar Cells. Hole Transfer as a Limiting Factor in Boosting Photoconversion Efficiency
Abstract: Semiconductor nanostructures are attractive for designing low cost solar cells with tunable photoresponse. The recent advances in size and shape selective synthesis have enabled the design of quantum dot solar cells with photoconversion efficiencies greater than 5%. In order to make them competitive with other existing thin film or polycrystalline photovoltaic technologies, it is important to overcome kinetic barriers for charge transfer at semiconductor interfaces. This feature focuses on the limitations imposed by slow hole transfer in improving solar cell performance and its role in the stability of metal chalcogenide solar cells. Strategies to improve the rate of hole transfer through surface modified redox relays offer new opportunities to overcome the hole transfer limitation. The mechanistic and kinetic aspects of hole transfer in Quantum Dot Solar Cells (QDSCs), Nanowire Solar Cells (NWSCs) and Extremely Thin Absorber (ETA) solar cells are discussed.
ACS Applied Materials and Interfaces - Cover Article
Congratulations to Doug Hines on a great cover art in the journal ACS Applied Materials and Interfaces! The cover art appeared in this month's issue of ACS Appl. Mater. Interfaces to go along with Doug's review of Quantum Dot Surface Chemistry entitled, Recent Advances in Quantum Dot Surface Chemistry.
About the Image: Size-dependent electronic and optical properties of semiconductor nanocrystals or quantum dots (QDs) make them attractive for designing photovoltaic, electronic, and optoelectronic devices. Because of a high surface-to-volume ratio, the optical behavior of QDs becomes sensitive to surface-bound species. Chemical modification of QD surfaces is thus a convenient approach to influence the excited state dynamics and charge transfer properties. The illustration shows how ligand exchange between organic molecules can modify the QD surface and thus their excited state properties. With increasing exchange, one can visually see the change in the emission properties of QDs.
Simple Tuning of CdSeS NWs
Read the latest paper from the Kamat Lab!
CdSeS Nanowires. Compositionally Controlled Band Gap and Exciton Dynamics

Abstract: CdS, CdSe and ternary CdSexS(1-x) are some of the most widely studied II-VI semiconductors due to their wide range of applications and promising performance in numerous systems. One-dimensional semiconductor nanowires offer the ability to conduct charges efficiently along the length of the wire which has potential charge transport benefits compared to nanoparticles. Herein, we report a simple, inexpensive synthetic procedure for high quality CdSeS nanowires where the composition can be easily modulated from pure CdSe to pure CdS by simply adjusting the Se:S precursor ratio. This allows for tuning of the absorption and emission properties of the nanowires across the visible spectrum. The CdSeS nanowires have a wurtzite crystal structure and grow along the [001] direction. As measured by femtosecond transient absorption spectroscopy, the short component of the excited state lifetime remains relatively constant at ~10 ps with increasing Se; however the contribution of this short lifetime component increased dramatically from 8.4% to 57.7% with increasing Se content. These CdSeS nanowires offer facile synthesis and widely adjustable optical properties, characteristics which give them broad potential applications in the fields of optoelectronics, and photovoltaics.
Publishing Scientific Research
Prashant and the other editors at the Journal of Physical Chemistry Letters have published a number of great editorials on scientific publishing. The topics range from scientific writing, to review, to multimedia.
- Overcoming the Myths of the Review Process and Getting Your Paper Ready for Publication
- The Increasing Impact of Multimedia and Social Media in Scientific Publications
- Increasing the Impact of Published Work. Introducing ACS Live Slides
- Getting your Submission Right and Avoiding Rejection
The editorials offer helpful insight into the scientific publishing process and help authors increase the impact of their work.
C&EN Cover Story - Perovskite Solar Cells

Chemical & Engineering News (C&EN) senior correspondant Mitch Jacoby recently visited our research group to talk about the exciting up and coming field of perovskite solar cells. Now, C&EN has run a great cover story article on this emerging research field. Dr. Kamat is quoted in the article and Joe Manser, a 3rd year Chemical and Biomolecular Engineering graduate student made the cover of the issue. You can read the full article on C&EN's website by following the link below.
"Every now and again, a well-studied research topic explodes with new life. Long after carbon materials filled chapters of dated textbooks, for example, that field's soul was reenergized around 1990 after buckyballs and carbon nanotubes were discovered. It happened again in that field about a half-dozen years ago when graphene took the world by storm. It's happening now in photovoltaics." - C&EN (image credit: Mitch Jacoby)
Excited State of [9]- and [12]-CPP
Read the latest paper from the Kamat Lab!
Carbon Nanohoops: Excited Singlet and Triplet Behavior of [9]- and [12]-cycloparaphenylene
![Carbon Nanohoops: Excited Singlet and Triplet Behavior of [9]- and [12]-cycloparaphenylene](images/News Pics/news83_CPPexcited.jpg)
Abstract: Cycloparaphenylene molecules, commonly known as 'carbon nanohoops,' have the potential to serve as building blocks in constructing carbon nanotube architectures. The singlet and triplet excited state characteristics of [9]-cycloparaphenylene ([9]CPP) and [12]-cycloparaphenylene ([12]CPP) have now been elucidated using time resolved transient absorption and emission techniques. The fluorescence quantum yields (Φ) of [9]CPP and [12]CPP were determined to be 0.46 and 0.83 respectively. Rates of non-radiative recombination (knr), radiative recombination (kr) and intersystem crossing (kisc) determined in this study indicate that radiative decay dominates in these nanohoop structures. The triplet quantum yields determined through energy transfer with excited biphenyl triplet were 0.18 and 0.13 for [9]CPP and [12]CPP respectively. The rate of triplet state quenching by oxygen was measured to be 1.7 × 103 s-1 ([9]CPP) and 1.9 × 103 s-1 ([12]CPP). The excited state dynamics established in this study enable us to understand the behavior of a carbon nanotube-like structure on a single subunit level.
Improved Charge Separation in CdSe QDSC with CuxS
Read the latest paper from the Kamat Lab!
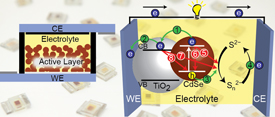
Charge Transfer Mediation Through CuxS. The Hole Story of CdSe in Polysulfide
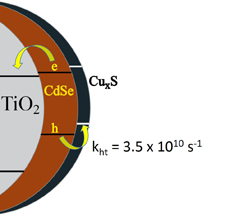
Abstract: Hole transfer to dissolved sulfide species in liquid junction CdSe quantum dot sensitized solar cells is relatively slow when compared to electron transfer from CdSe to TiO2. Controlled exposure of cadmium chalcogenide surfaces to copper ions followed by immersion in sulfide solution promotes development of interfacial CuxS layer, which mediates hole transfer to polysulfide electrolyte by collection of photogenerated holes from CdSe. In addition, CuxS was also found to interact directly with defect states on the CdSe surface and quench emission characteristic of electron traps resulting from selenide vacancies. Together these effects were found to work in tandem to deliver 6.6% power conversion efficiency using Mn-doped CdS and CdSe co-sensitized quantum dot solar cell. Development of n-p interfacial junction at the photoanode-electrolyte interface in quantum dot solar cells unveils new means for designing high efficiency liquid junction solar cells.
Quantum Dot Surface Chemistry
Read the latest paper from the Kamat Lab!
Recent Advances in Quantum Dot Surface Chemistry
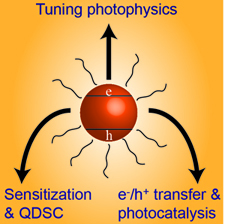
Abstract: Quantum dot (QD) surface chemistry is an emerging field in semiconductor nanocrystal related research. Along with size manipulation, the careful control of QD surface chemistry allows modulation of the optical properties of a QD suspension. Even a single molecule bound to the surface can introduce new functionalities. Herein, we summarize the recent advances in QD surface chemistry and the resulting effects on optical and electronic properties. Specifically, the feature article focuses addresses three main issues: (i) How surface chemistry affects the optical properties of QDs, (ii) How it influences the excited state dynamics and (iii) How one can manipulate surface chemistry to control the interactions between QDs and metal oxides, metal nanoparticles and in self-assembled QD monolayers.
Webinar - Rate Limiting Interfacial Hole Transfer in Sb2S3
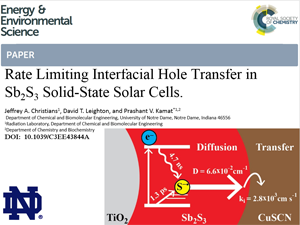
Watch this webinar on the hole transfer process between Sb2S3 and CuSCN. The hole diffusion coefficient and diffusion length in Sb2S3 is measured. Hole transfer from Sb2S3 to CuSCN is found to be predominately limited by transfer across the Sb2S3–CuSCN interface. It is based off of the paper, recently published in the Energy and Environmental Science, entitled:
Rate Limiting Interfacial Hole Transfer in Sb2S3 Solid-State Solar Cells.Abstract: Transfer of photogenerated holes from the absorber species to the p-type hole conductor is fundamental to the performance of solid-state sensitized solar cells. In this study, we comprehensively investigate hole diffusion in the Sb2S3 absorber and hole transfer across the Sb2S3–CuSCN interface in the TiO2/Sb2S3/CuSCN system using femtosecond transient absorption spectroscopy, carrier diffusion modeling, and photovoltaic performance studies. Transfer of photogenerated holes from Sb2S3 to CuSCN is found to be dependent on Sb2S3 film thickness, a trend attributed to diffusion in the Sb2S3 absorber. However, modeling reveals that this process is not adequately described by diffusion limitations alone as has been assumed in similar systems. Therefore, both diffusion and transfer across the Sb2S3–CuSCN interface are taken into account to describe the hole transfer dynamics. Modeling of diffusion and interfacial hole transfer effects reveal that interfacial hole transfer, not diffusion, is the predominate factor dictating the magnitude of the hole transfer rate, especially in thin (< 20 nm) Sb2S3 films. Lastly, the implications of these results are further explored by photovoltaic measurements using planar TiO2/Sb2S3/CuSCN solar cells to elucidate the role of hole transfer in photovoltaic performance.
Driving Charge Separation in Hybrid Materials
Read the latest paper from the Kamat Lab!
Driving Charge Separation for Hybrid Solar Cells: Photo-induced Hole Transfer in Conjugated Copolymer and Semiconductor Nanoparticle Assemblies
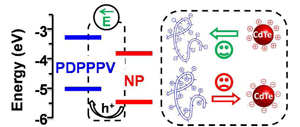
Abstract: This work reports on the use of an internal electrostatic field to facilitate charge separation at inorganic-organic interfaces, analogous to those in hybrid solar cells. Systematic charge transfer studies show that the donor-acceptor charge transfer rate is highly sensitive to the direction of the internal electric field.
Hole Transfer and Diffusion in Sb2S3
Read the latest paper from the Kamat Lab!
Rate Limiting Interfacial Hole Transfer in Sb2S3 Solid-State Solar Cells
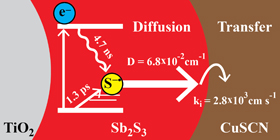
Abstract: Transfer of photogenerated holes from the absorber species to the p-type hole conductor is fundamental to the performance of solid-state sensitized solar cells. In this study, we comprehensively investigate hole diffusion in the Sb2S3 absorber and hole transfer across the Sb2S3–CuSCN interface in the TiO2/Sb2S3/CuSCN system using femtosecond transient absorption spectroscopy, carrier diffusion modeling, and photovoltaic performance studies. Transfer of photogenerated holes from Sb2S3 to CuSCN is found to be dependent on Sb2S3 film thickness, a trend attributed to diffusion in the Sb2S3 absorber. However, modeling reveals that this process is not adequately described by diffusion limitations alone as has been assumed in similar systems. Therefore, both diffusion and transfer across the Sb2S3–CuSCN interface are taken into account to describe the hole transfer dynamics. Modeling of diffusion and interfacial hole transfer effects reveal that interfacial hole transfer, not diffusion, is the predominate factor dictating the magnitude of the hole transfer rate, especially in thin (< 20 nm) Sb2S3 films. Lastly, the implications of these results are further explored by photovoltaic measurements using planar TiO2/Sb2S3/CuSCN solar cells to elucidate the role of hole transfer in photovoltaic performance.
Aqueous •OH Mediated Degradation of Dimethyl Phthalate
Read the latest paper from the Kamat Lab!
Kinetics and Mechanism of •OH Mediated Degradation of Dimethyl Phthalate in Aqueous Solution: Experimental and Theoretical Studies
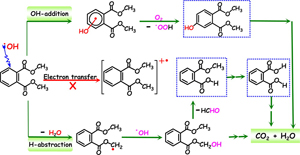
Abstract: The hydroxyl radical (•OH) is one of the main oxidative species in aqueous phase advanced oxidation processes, and its initial reactions with organic pollutants are important to understand the transformation and fate of organics in water environments. Insights into the kinetics and mechanism of •OH mediated degradation of the model environmental endocrine disruptor, dimethyl phthalate (DMP), have been obtained using radiolysis experiments and computational methods. The bimolecular rate constant for the •OH reaction with DMP was determined to be (3.2 ± 0.1) × 109 M–1s–1. The possible reaction mechanisms of radical adduct formation (RAF), hydrogen atom transfer (HAT), and single electron transfer (SET) were considered. By comparing the experimental absorption spectra with the computational results, it was concluded that the RAF and HAT were the dominant reaction pathways, and OH-adducts (•DMPOH1, •DMPOH2) and methyl type radicals •DMP(-H)α were identified as dominated intermediates. Computational results confirmed the identification of transient species with maximum absorption around 260 nm as •DMPOH1 and •DMP(-H)α, and these radical intermediates then converted to monohydroxylated dimethyl phthalates and monomethyl phthalates. Experimental and computational analyses which elucidated the mechanism of •OH-mediated degradation of DMP are discussed in detail.
Phys.org Highlights Perovskite Solar Cells
Lisa Zyga of the website Phys.org has written a great piece highlighting the copper iodide based perovskite solar cells recently developed in our lab. The entire article can be found at their website by following the link below.
Perovskite solar cells become even more promising with cheaper materials "(Phys.org) — Due to their rapid improvements in a short amount of time, perovskite solar cells have become one of today's most promising up-and-coming photovoltaic technologies. Currently, the record efficiency for a perovskite solar cell is 15% and expected to improve further. Although the perovskite material itself is relatively inexpensive, the best devices commonly use an expensive organic hole-conducting polymer, called spiro-OMeTAD, which has a commercial price that is more than 10 times that of gold and platinum."
Updated Research Pages

Our research focuses on the fields of alternative energy and environmental research, with a special emphasis on an understanding of the fundamental science behing these processes which guides our development of improved devices for real world applications. We seek to build better devices by better understanding how they work.
View an updated summary of all of the ongoing research in the Kamat Lab on our research pages. We provide a brief description of our field and the specific challenges that we seek to address in our lab along with a description of recent developments from our research and select publications. Visit all of the different research pages to get a full overview of our current work!
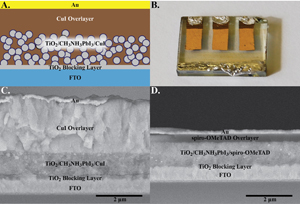
Webinar - Copper Iodide-Lead Iodide Perovskite Solar Cells

Watch this webinar on an inorganic hole conductor for the emerging perovskite solar cells with efficiencies as high as 6% compared to 7.9% with sprio-OMeTAD. It is based off of the paper, recently published in the Journal of the American Chemical Society, entitled:
An Inorganic Hole Conductor for Organo-Lead Halide Perovskite Solar Cells. Improved Hole Conductivity with Copper Iodide.Abstract: Organo-lead halide perovskite solar cells have emerged as one of the most promising candidates for the next generation of solar cells. To date, these perovskite thin film solar cells have exclusively employed organic hole conducting polymers which are often expensive and have low hole mobility. In a quest to explore new inorganic hole conducting materials for these perovskite based thin film photovoltaics, we have identified copper iodide as a possible alternative. Using copper iodide, we have succeeded in achieving a promising power conversion efficiency of 6.0% with excellent photocurrent stability. The open-circuit voltage, compared to the best spiro-OMeTAD devices, remains low and is attributed to higher recombination in CuI devices as determined by impedance spectroscopy. However, impedance spectroscopy revealed that CuI exhibits two orders of magnitude higher electrical conductivity than spiro-OMeTAD which allows for significantly higher fill factors. Reducing the recombination in these devices could render CuI as a cost effective competitor to spiro-OMeTAD in perovskite solar cells.
Kamat Group 2013 Annual Report

2013 has been a very productive year for the Kamat group. Our group said goodbye to Dr. Sean Murphy who successfully defended his thesis and received a postdoc at the University of Melbourne, as well as postdocs Ganganahalli Ramesha, and Pralay Santra. Three new graduate students joined the lab, Seogjoon Yoon, Victoria Bridewell, and Steven Kobosko, as well as two postdocs, Rabeka Alam, and Kevin Stamplecoskie. Prashant received numerous awards and recognitions, including the Langmuir Lectureship Award, gave invited presentations at 8 universities around the world, and gave 19 presentations at 13 different conferences! Definitely a lot of traveling this year! As a group, the research in the Kamat lab continues to be highly productive with Prashant's h-index surpassing 100 this year and 18 new publications. As a group, we would like to extend our thanks to all those who have contributed to making this research group successful! Best wishes for a happy and prosperous new year!
Excited State Dynamics of Au Clusters
Read the latest paper from the Kamat Lab!
Excited State Behavior of Luminescent Glutathione Protected Gold Clusters
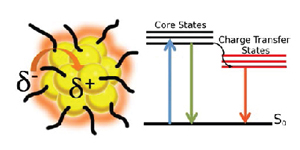
Abstract: The excited state behavior of luminescent gold clusters provide new insights in understanding their photocatalytic activity in the visible region. The excited state of glutathione protected gold nanoclusters (AuGSH) which is characterized by the long-lived excited state (τ = 780 ns) arises from the ligand-to-metal type transition. These AuGSH clusters are in a partially oxidized state, (Au(I))and are readily reduced by chemical or electrochemical methods. Interestingly a metal core transition with short lived lifetime (τ <3 ps) appears along with a longer lifetime in reduced AuGSH clusters. The role of oxidation state of gold clusters in dictating the photocatalytic reduction of methyl viologen is discussed.
Perovskite Solar Cells with Inorganic Hole Conductor
Read the latest paper from the Kamat Lab!
An Inorganic Hole Conductor for Organo-Lead Halide Perovskite Solar Cells. Improved Hole Conductivity with Copper Iodide
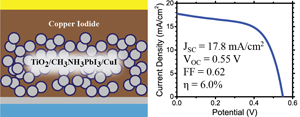
Abstract: Organo-lead halide perovskite solar cells have emerged as one of the most promising candidates for the next generation of solar cells. To date, these perovskite thin film solar cells have exclusively employed organic hole conducting polymers which are often expensive and have low hole mobility. In a quest to explore new inorganic hole conducting materials for these perovskite based thin film photovoltaics, we have identified copper iodide as a possible alternative. Using copper iodide, we have succeeded in achieving a promising power conversion efficiency of 6.0% with excellent photocurrent stability. The open-circuit voltage, compared to the best spiro-OMeTAD devices, remains low and is attributed to higher recombination in CuI devices as determined by impedance spectroscopy. However, impedance spectroscopy revealed that CuI exhibits two orders of magnitude higher electrical conductivity than spiro-OMeTAD which allows for significantly higher fill factors. Reducing the recombination in these devices could render CuI as a cost effective competitor to spiro-OMeTAD in perovskite solar cells.
Direct Observation of GO Reduction Kinetics
Read the latest paper from the Kamat Lab!
Direct Observation of Spatially Heterogeneous Single-Layer Graphene Oxide Reduction Kinetics
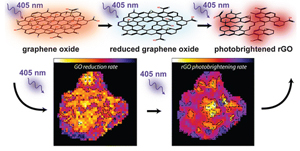
Abstract: Graphene oxide (GO) is an important precursor in the production of chemically derived graphene. During reduction, GO's electrical conductivity and band gap change gradually. Doping and chemical functionalization are also possible, illustrating GO's immense potential in creating functional devices through control of its local hybridization. Here we show that laser-induced photolysis controllably reduces individual single-layer GO sheets. The reaction can be followed in real time through sizable decreases in GO's photoluminescence efficiency along with spectral blueshifts. As-produced reduced graphene oxide (rGO) sheets undergo additional photolysis, characterized by dramatic emission enhancements and spectral redshifts. Both GO's reduction and subsequent conversion to photobrightened rGO are captured through movies of their photoluminescence kinetics. Rate maps illustrate sizable spatial and temporal heterogeneities in sp2 domain growth and reveal how reduction "flows" across GO and rGO sheets. The observed heterogeneous reduction kinetics provides mechanistic insight into GO's conversion to chemically derived graphene and highlights opportunities for overcoming its dynamic, chemical disorder.
CdSe/CdS Nanowire Solar Cells
Read the latest paper from the Kamat Lab!
Sequentially Layered CdSe/CdS Nanowire Architecture for Improved Nanowire Solar Cell Performance
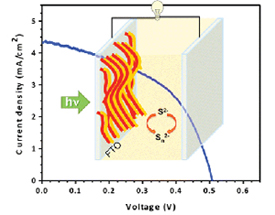
Abstract: The power conversion efficiency of semiconductor nanowire (NW) based solar cells as compared to quantum dot solar cell (QDSC) has remained lower, and efforts to improve the photovoltaic performance of semiconductor NWs continue. We have now succeeded in using a layered architecture of CdS and CdSe NWs for improving the photovoltaic performance of nanowire solar cell (NWSC). The photoanode designed with sequentially deposited films of CdSe and CdS NWs delivered a power conversion efficiency of 1%. This efficiency of CdSe/CdS composite is an order of magnitude improvement over single nanowire system (CdS or CdSe) based solar cell. The improvement seen in the CdSe/CdS composite film is attributed to charge rectification and improvement of electron and hole separation and transport in the opposite direction. Impedance spectroscopy demonstrates the beneficial effect of type II structure in CdSe/CdS sequential deposition through lower transport resistance, which remains a dominating effect in dictating the overall performance of the NWSC.
Eilers Fellowship Winner - Jeff Christians

Jeff Christians, a graduate student in our lab, was recently awarded the Patrick and Jana Eilers Graduate Student Fellowship for Energy Related Research on behalf of the center for sustainable energy at Notre Dame. This fellowship allows Jeff to further explore the emerging area of perovskite solar cells. Jeff will be working on understanding the hole transfer processes in these devices, and working to fabricate higher efficiency solar cells. Congratulations to Jeff for this award!
Farewell Dr. Jong-Pil Kim

Dr. Jong-Pil Kim spent the past 9 months researching in the Kamat lab. Dr. Kim came to the University of Notre Dame as a visiting researcher from the Busan Center at the Korea Basic Science Institute in South Korea. During his time at Notre Dame, Dr. Kim studied the synthesis of CdSe, CdS and CdSeS nanowires for photovoltaic applications and greatly improved his English. As a group, we would like to thank Dr. Jong-Pil Kim for his time spent at Notre Dame and for his valuable contributions to our group!
Testing Fruit-Sensitized Solar Cells

About 20 sophomore students at Notre Dame test dye-sensitized solar cells they made in lab using blueberry, raspberry, blackberry, or tea as the light absorber. Joe Manser, a third year chemical engineering graduate student in our lab, gave the students an introduction on how DSSCs work and then took them up into our lab and helped them measure the solar cells they made. This lab was developed in conjuction with Ken Poling, a science teacher at Penn High School in South Bend, through the Research Experience for Teachers (RET) program this past summer. Joe Manser and Jeff Christians worked with Ken to develop the techniques needed to make fruit-sensitized solar cells in the summer, and then helped the students as they measured their solar cell efficiency. It was a really great experience to help teach students about solar energy!
CdS Nanowire Solar Cells and Squaraine
Read the latest paper from the Kamat Lab!
CdS Nanowire Solar Cells: Dual Role of Squaraine Dye as a Sensitizer and a Hole Transporter
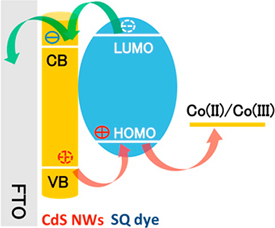
Abstract: The squaraine dye (SQ) anchored onto CdS nanowires serves as a photosensitizing dye and a hole acceptor. This dual role of the squaraine dye has been successfully exploited in a nanowire solar cell to improve the photoconversion efficiency. Electrophoretic deposition of CdS NWs and CdS NWs+SQ composite onto conducting glass electrodes was performed to obtain robust photoanodes and evaluate the photovoltaic performance of nanowire solar cells (NWSCs). Whereas the sensitization property of the SQ extends the response of CdS NWSCs into the near-IR (NIR) region, its redox property facilitates shuttling of holes to the electrolyte and suppressing the charge recombination process. Transient absorption measurements confirm the formation of cation radical of the dye arising from these two processes. The dual role of the squaraine dye has enabled us to improve the power conversion efficiency of NWSCs by a factor of ~20. Photoelectrochemical, spectroelectrochemical, and spectroscopic measurements provide insight into the multifaceted role of squaraine dye in improving the performance of NWSCs.
Kamat Lab Twitter: @KamatLabND

We now have a Kamat Lab twitter page! Follow the Kamat Lab on Twitter @KamatLabND. Find interesting research articles about different areas of science our lab is interested in and other papers that grabbed our attention. In addition, keep up to date with our latest publications and group news. Feel free to tweet us if you come across anything interesting, we'd love to hear from you!
You can find our Twitter page in the future by reminding to follow us or by simply clicking the blue Twitter bird on the right side of the navigation bar at the top of each page.
Prashant Kamat - 30 years at ND

This year, Prashant is celebrating 30 years at the University of Notre Dame in the Radiation Laboratory. To help him celebrate, we planned a surprise cookout lunch for the entire Radiation Laboratory staff. Doug and Jeff grilled hamburgers and bratwursts (after taking an online food safety course), and James set up a fake conference call meeting with Prashant then lured him outside with a "safety hazard" that he wanted checked out. We had beautiful weather outside and great food!
Nickel-Doped MnO2 for Lithium Batteries
Read the latest paper from the Kamat Lab!
Nickel-Doped MnO2 Nanowires Anchored onto Reduced Graphene Oxide for Rapid Cycling Cathode in Lithium Ion Batteries

Abstract: Nickel-doped MnO2 nanowires were synthesized directly onto reduced graphene oxide (RGO) to generate a composite cathode material with improved high-rate cycling characteristics. The presence of RGO improves the electrochemical characteristics of the cathode in Li-ion half-cell architecture. Cyclic voltammetry, electrochemical impedance spectroscopy, and electrode cycling are confirm that RGO plays a major role in enhancing the ability of the NixMn(1-x)O2 to reversibly intercalate lithium ions at 1C rate. The chronocoulometric response of the RGO-based electrode shows the improvements originate from faster reaction kinetics and transport of Li+ coupled with increased specific capacitance and Li+ adsorption.
Prashant Awarded 2013 Langmuir Lectureship

Congratulations to Prashant on receiving the 2013 Langmuir Lectureship! Prashant gave his lecture entitled, "Meeting the Clean Energy Challenge with Semiconductor Nanostructures" at the 2013 ACS National Meeting in Indianapolis, Indiana. Prashant's lecture was so well attended that chairs had to be brought in from the neighboring lecture hall and there was still a large group standing in the back (and even out the back!) of the lecture hall. Prashant was one of two recipients of the award, along with Dr. Nicholas A. Kotov from the University of Michigan, which was co-sponsored by the ACS Division of Colloid & Surface Chemistry and the journal Langmuir. Join with us in the Kamat group in congratulating Prashant on this wonderful career achievement!
Webinar - Sb2S3 Hole Transfer

Watch this webinar on the transfer of holes from Sb2S3 to CuSCN. This webinar describes the experimental results and the observed mechanism of the hole transfer. It is based off of the paper, recently published in ACS Nano, entitled:
Trap and Transfer. Two-Step Hole Injection Across the Sb2S3/CuSCN Interface in Solid State Solar Cells.Abstract: In solid-state semiconductor-sensitized solar cells, commonly known as extremely thin absorber (ETA) or solid-state quantum dot sensitized solar cells (QDSCs), transfer of photogenerated holes from the absorber species to the p-type hole conductor plays a critical role in the charge separation process. Using Sb2S3 (absorber) and CuSCN (hole conductor), we have constructed ETA solar cells exhibiting a power conversion efficiency of 3.3%. The hole transfer from excited Sb2S3 into CuSCN, which limits the overall power conversion efficiency of these solar cells, is now independently studied using transient absorption spectroscopy. In the Sb2S3 absorber layer, photogenerated holes are rapidly localized on the sulfur atoms of the crystal lattice, forming a sulfide radical (S−•) species. This trapped hole is transferred from the Sb2S3 absorber to the CuSCN hole conductor with an exponential time constant of 1680 ps. This process was monitored through the spectroscopic signal seen for the S−• species in Sb2S3, providing direct evidence for the hole transfer dynamics in ETA solar cells. Elucidation of the hole transfer mechanism from Sb2S3 to CuSCN represents a significant step toward understanding charge separation in Sb2S3 solar cells, and provides insight into the design of new architectures for higher efficiency devices.
Sb2S3 Hole Transfer
Read the latest paper from the Kamat Lab!
Trap and Transfer. Two-Step Hole Injection Across the Sb2S3/CuSCN Interface in Solid State Solar Cells.
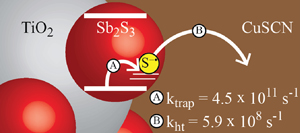
Abstract: In solid-state semiconductor-sensitized solar cells, commonly known as extremely thin absorber (ETA) or solid-state quantum dot sensitized solar cells (QDSCs), transfer of photogenerated holes from the absorber species to the p-type hole conductor plays a critical role in the charge separation process. Using Sb2S3 (absorber) and CuSCN (hole conductor), we have constructed ETA solar cells exhibiting a power conversion efficiency of 3.3%. The hole transfer from excited Sb2S3 into CuSCN, which limits the overall power conversion efficiency of these solar cells, is now independently studied using transient absorption spectroscopy. In the Sb2S3 absorber layer, photogenerated holes are rapidly localized on the sulfur atoms of the crystal lattice, forming a sulfide radical (S−•) species. This trapped hole is transferred from the Sb2S3 absorber to the CuSCN hole conductor with an exponential time constant of 1680 ps. This process was monitored through the spectroscopic signal seen for the S−• species in Sb2S3, providing direct evidence for the hole transfer dynamics in ETA solar cells. Elucidation of the hole transfer mechanism from Sb2S3 to CuSCN represents a significant step toward understanding charge separation in Sb2S3 solar cells, and provides insight into the design of new architectures for higher efficiency devices.
Quantum Dot Surface Chemistry
Read the latest paper from the Kamat Lab!
Quantum Dot Surface Chemistry: Ligand Effects and Electron Transfer Reactions.
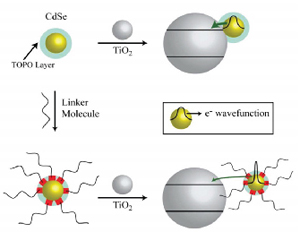
Abstract: With the increased interest in quantum dot sensitized solar cells (QDSCs) there comes a need to better understand how surface modification of quantum dots (QDs) can affect the excited state dynamics of QDs, electron transfer at the QD-metal oxide (MO) interface, and overall photoconversion efficiency of QDSCs. We have monitored the surface modification of solution based QDs via the steady-state absorption and emission characteristics of colloidal CdSe passivated with β-Alanine (β-Ala). The trap-remediating nature of the β-Ala molecule, arising from the Lewis-basicity of the amine group, is realized from the hypsochromic shifts seen in excitonic absorption and emission bands as well as an increase in fluorescence quantum yield. Transient absorption measurements of CdSe-TiO2 films prepared with and without β-Ala as a linker molecule further reveal the role of the surface modifier in influencing excited state electron transfer processes. Electron transfer at this interface was dependent on the method of QD deposition: CdSe-TiO2 (direct deposition, ket = 1.5 × 10^10 s-1), CdSe-linker-TiO2 (attaching linker molecule first to TiO2 so that β-Ala interaction is minimal, ket = 2.4 × 10^9 s-1) or linker-CdSe-linker-TiO2 (linkage via full β-Ala encapsulation in solution prior to deposition, ket = 6.4 × 10^8 s-1). These results imply that the surface chemistry of colloidal CdSe plays an important role in mediating electron transfer reactions.
Metal-Cluster-Sensitized Solar Cells
Read the latest paper from the Kamat Lab!
Metal-Cluster-Sensitized Solar Cells. A New Class of Thiolated Gold Sensitizers Delivering Efficiency Greater Than 2%.
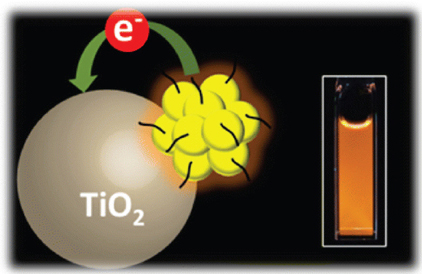
Abstract: A new class of metal-cluster sensitizers has been explored for designing high-efficiency solar cells. Thiol-protected gold clusters which exhibit molecular-like properties have been found to inject electrons into TiO2 nanostructures under visible excitation. Mesoscopic TiO2 films modified with gold clusters deliver stable photocurrent of 3.96 mA/cm2 with power conversion efficiencies of 2.3% under AM 1.5 illumination. The overall absorption features and cell performance of metal-cluster-sensitized solar cells (MCSCs) are comparable to those of CdS quantum-dot-based solar cells (QDSCs). The relatively high open-circuit voltage of 832 mV and fill factor of 0.7 for MCSCs as compared to QDSCs show the viability of these new sensitizers as alternatives to semiconductor QDs and sensitizing dyes in the next generation of solar cells. The superior performance of MCSCs discussed in this maiden study lays the foundation to explore other metal clusters with broader visible absorption.
Perspective Article - Energy Outlook for Planet Earth
Read the latest paper from the Kamat Lab!
Energy Outlook for Planet Earth
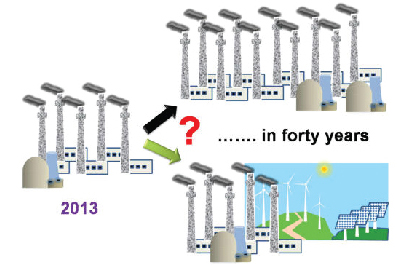
Growing economies and increasing population will necessitate an increased energy supply in the coming years. A recent BP report of the 2030 Energy Outlook suggests that an additional 1.3 billion people will become new energy consumers by 2030. If the current energy growth of 1.6% per year continues, this would require the energy production to double in about 40 years. In fact, Exxon's 2040 Energy Outlook projects an 85% increase in global electricity demand over the period of 2010–2040. Developing (nonOECD) countries alone will experience a 150% surge in electricity demand. The sheer magnitude of this energy demand will require us to make some tough choices: either continue to build the same number of coal and nuclear power plants or explore the production of renewable energy coupled with improved efficiency of energy usage (Figure).
Alex Mobashery 4th in International Science Fair

Alex Mobashery, a Penn High School, Mishawaka student carried out research in the Kamat laboratory for the past two years (his junior and senior year). Recently, he participated in the Intel International Science and Engineering Fair 2013.
His Project "Boosting Current of Quantum Dot Sensitized Solar Cells with CdS/PbS Heterostructures" placed fourth in the Energy and Transportation category of the Grand Award Winner.
Join us in congratulating Alex on his great work in our lab and awesome showing at the Science and Engineering Fair!
Fellowship Winner - James Radich

James Radich, a graduate student in our lab, was recently awarded two fellowships that allow him to explore his own proposed research topics: the Eiler's Sustainable Energy Research Fellowship and the Bayer Environmental Research Fellowship. The energy research will focus on the incorporation of copper-based p-type nanocrystals into solid state quantum dot solar cells. The environmental research pivots from a recent publication on Holey Graphene and aims to understand the fate of graphene and other carbon nanomaterials in oxidative environments. Congratulations to James for these awards!
Webinar - Making Graphene Holey

Watch this webinar the formation of holes in graphene due to gold-nanoparticle-mediated attack of the reduced graphene oxide sheet by hydroxyl radicals. This webinar is based off of the paper, recently published in ACS Nano, entitled:
Making Graphene Holey. Gold-Nanoparticle-Mediated Hydroxyl Radical Attack on Reduced Graphene OxideAbstract: Graphene oxide (GO) and reduced graphene oxide (RGO) have important applications in the development of new electrode and photocatalyst architectures. Gold nanoparticles (AuNPs) have now been employed as catalyst to generate OH• and oxidize RGO via hydroxyl radical attack. The oxidation of RGO is marked by pores and wrinkles within the 2-D network. Nanosecond laser flash photolysis was used in conjunction with competition kinetics to elucidate the oxidative mechanism and calculate rate constants for the AuNP-catalyzed and direct reaction between RGO and OH•. The results highlight the use of the AuNP-mediated oxidation reaction to tune the properties of RGO through the degree of oxidation and/or functional group selectivity in addition to the nanoporous and wrinkle facets. The ability of AuNPs to catalyze the photolytic decomposition of H2O2 as well as the hydroxyl radical-induced oxidation of RGO raises new issues concerning graphene stability in energy conversion and storage (photocatalysis, fuel cells, Li-ion batteries, etc.). Understanding RGO oxidation by free radicals will aid in maintaining the long-term stability of RGO-based functional composites where intimate contact with radical species is inevitable.
Webinar - Galvanic Exchange on Reduced Graphene Oxide
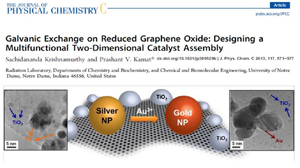
Watch this webinar on how to promote galvinic exchange on the surface of reduced graphene oxide for the design of new catalyst materials. This webinar is based off of the paper, published in the Journal of Physical Chemistry C, entitled:
Galvanic Exchange on Reduced Graphene Oxide. Designing a Multifunctional Two-Dimensional Catalyst Assembly.Abstract: The two-dimensional network of reduced graphene oxide (RGO) is decorated with silver and gold nanoparticles. The silver nanoparticles deposited on RGO by photocatalytic reduction are subjected to galvanic exchange with Au3+ ions to transform them into gold nanoparticles. This compositional change on the RGO surface demonstrates RGO's versatile ability to anchor a wide array of nano-particles and facilitate chemical transformations. Coupled with RGO's unique ability to capture and transport electrons, galvanic exchange is used to contrive a two-dimensional nano catalyst mat. Raman studies show that metal nanoparticles anchored on reduced graphene oxide facilitate enhancement of Raman bands. Using methyl viologen as a probe we elucidate the photocatalytic activity of the Semiconductor-RGO-Metal nanoassembly and highlight the mediation of RGO in charge transfer processes.
Making Graphene Holy Holey
Read the latest paper from the Kamat Lab!
Making Graphene Holey. Gold-Nanoparticle-Mediated Hydroxyl Radical Attack on Reduced Graphene Oxide
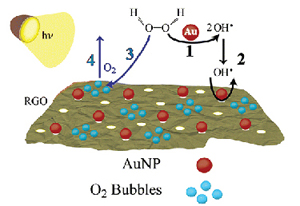
Abstract: Graphene oxide (GO) and reduced graphene oxide (RGO) have important applications in the development of new electrode and photocatalyst architectures. Gold nanoparticles (AuNPs) have now been employed as catalyst to generate OH• and oxidize RGO via hydroxyl radical attack. The oxidation of RGO is marked by pores and wrinkles within the 2-D network. Nanosecond laser flash photolysis was used in conjunction with competition kinetics to elucidate the oxidative mechanism and calculate rate constants for the AuNP-catalyzed and direct reaction between RGO and OH•. The results highlight the use of the AuNP-mediated oxidation reaction to tune the properties of RGO through the degree of oxidation and/or functional group selectivity in addition to the nanoporous and wrinkle facets. The ability of AuNPs to catalyze the photolytic decomposition of H2O2 as well as the hydroxyl radical-induced oxidation of RGO raises new issues concerning graphene stability in energy conversion and storage (photocatalysis, fuel cells, Li-ion batteries, etc.). Understanding RGO oxidation by free radicals will aid in maintaining the long-term stability of RGO-based functional composites where intimate contact with radical species is inevitable.
