News From the Kamat Lab
CdSe-CdS Nanowire Solar Cells
Read the latest paper from the Kamat Lab!
Improved Performance of CdSe Nanowire Solar Cells using Carbazole as Surface Modifier.
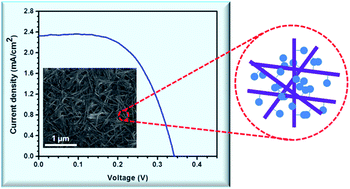
Abstract: Carbazole molecules containing thiol functional groups, when attached to CdSe nanowires (NWs), facilitate hole transport across semiconductor interfaces. The improved hole transfer rate is evidenced by increased electron lifetimes and better photovoltaic performance. Nanowire solar cells (NWSCs) with carbazole treatment delivered a power conversion efficiency of 0.46%, which is an order of magnitude improvement over untreated films. The illumination of the sample during the electrophoretic deposition of nanowires also had a profound effect in obtaining stable and higher photocurrents.
Dr. Sean Murphy

Congratulations to Dr. Sean Murphy on successful completion of his Ph. D. in Chemistry! Sean successfully defended his doctoral thesis entitled: Metal Nanoparticle-Graphene Oxide Composites: Photophysical Properties and Sensing Applications. Congratulations on completion of your Ph. D. Sean!
Video - Making Quantum Dot Solar Cells
Watch the J. Phys. Chem. Lett. Video Perspective
Recent advances seen in the utilization of semiconductor nanostructures for light energy conversion as well as the future prospects of quantum dot solar cells (QDSCs) provides the basis for QDSCs consideration as "The Next Big Thing" in photovoltaics. In this video perspective - based on Dr. Kamat's latest Journal of Physical Chemistry Letters perspective article "Quantum Dot Solar Cells. The Next Big Thing in Photovoltaics." (DOI: 10.1021/jz400052e) - Joe Manser, a second year chemical engineering graduate student from our group, shows the step-by-step process of making high efficiency QDSCs.
The Next Big Thing in Photovoltaics
Read the latest paper from the Kamat Lab!
Quantum Dot Solar Cells. The Next Big Thing in Photovoltaics
Abstract: The recent surge in the utilization of semiconductor nanostructures for solar energy conversion has led to the development of high-efficiency solar cells. Some of these recent advances are in the areas of synthesis of new semiconductor materials and the ability to tune the electronic properties through size, shape, and composition and to assemble quantum dots as hybrid assemblies. In addition, processes such as hot electron injection, multiple exciton generation (MEG), plasmonic effects, and energy-transfer-coupled electron transfer are gaining momentum to overcome the efficiency limitations of energy capture and conversion. The recent advances as well as future prospects of quantum dot solar cells discussed in this perspective provide the basis for consideration as "The Next Big Thing" in photovoltaics.
Congratulations Alex Mobashery - 1st Place NIRSEF

Congratulations to Alex Mobashery (a high school student who has spent the past two years working weekends and summers in our lab) who finished 1st place in the Energy and Transportation category in the Northern Indiana Regional Science and Engineering Fair!
Alex was awarded the Exposition Award for excellence in the study of energy, and the Notre Dame Physics Club Award!
He also qualified to compete (and has accommodations paid for!) in the International Sustainable World Project Olympiad in Houston, Texas, and the 2013 Hoosier Science and Engineering Fair in Indianapolis, Indiana!
Congratulations Alex on all the awards and invitations!
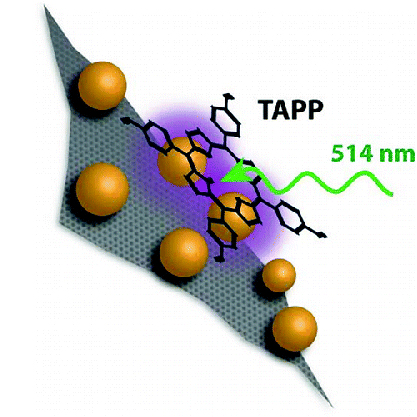
RGO-Ag Composite as SERS Material
Read the latest paper from the Kamat Lab!
Reduced Graphene Oxide–Silver Nanoparticle Composite as an Active SERS Material
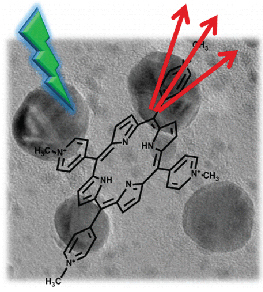
Abstract: Selectivity and enhanced sensitivity for SERS measurements are highly desirable for environmental and analytical applications. Interaction of a target molecule with SERS substrate plays a pivotal role in determining the magnitude of enhancement and spectral profile of the SERS signal. A reduced graphene oxide–Ag nanoparticle (RGO-Ag NP) composite has been designed to boost SERRS sensitivity of a porphyrin derivative. Complexation between 5,10,15,20-tetrakis(1-methyl-4-pyridinio)porphyrin tetra(p-toluenesulfonate) (TMPyP) porphyrin and the RGO-Ag NP composite is evidenced by a red-shifted porphyrin absorption band. Results indicate complexation is influential in improved surface-enhanced resonance Raman (SERRS) signal for TMPyP and thus offers an advantage for target molecule detection at low concentration levels. The combined effects of RGO and Ag NPs in the enhancement of SERS signal of TMPyP are discussed.
CuInS Quantum Dot Solar Cells
Read the latest paper from the Kamat Lab!
CuInS2-Sensitized Quantum Dot Solar Cell. Electrophoretic Deposition, Excited-State Dynamics, and Photovoltaic Performance
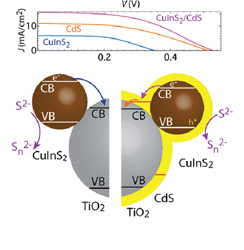
Abstract: Ternary metal chalcogenides such as CuInS2 offer new opportunities to design quantum dot solar cells (QDSC). Chemically synthesized CuInS2 quantum dots (particle diameter, 2.6 nm) have been successfully deposited within the mesoscopic TiO2 film using electrophoretic deposition (150 V cm–1 dc field). The primary photoinduced process of electron injection from excited CuInS2 into TiO2 occurs with a rate constant of 5.75 × 1011 s–1. The TiO2/CuInS2 films are photoactive and produce anodic photocurrent with a power conversion efficiency of 1.14%. Capping the TiO2/CuInS2 film with a CdS layer decreases the interfacial charge recombination and thus offers further improvement in the power conversion efficiency (3.91%). The synergy of using CdS as a passivation layer in the composite film is also evident from the increased external quantum efficiency of the electrode in the red region where only CuInS2 absorbs the incident light.
Webinar - Tandem-Layered QDSCs
View our latest Slideshare webinar (slides with audio) entitled:
Tandem Layered Quantum Dot Solar Cells Tuning the Photovoltaic Response with Luminescent Ternary Cadmium Chalcogenides
This webinar highlights some of Dr. Pralay Santra and Dr. Prashant Kamat's recent work showing the improvement of QDSCs employing a layered structure with CdSeS quantum dots due to the synergistic effects of layering.
2012 Annual Research Summary

2012 was a great year in the Kamat Lab! Ian Lightcap, Ben Meekins, and Matt Becker all recieved their Ph. D. and went on to positions at the center for sustainable energy at Notre Dame (cSEND), University of Texas, Austin as a postdoc under Dr. Bard, and a teaching position at the Roechester Institute of Technology, respectively. Three new Ph. D. students joined the lab, Jacob Hoffman (Chemistry), Danilo JaraQuinteros (Chemistry), and Anshumaan Bajapai. As a group, we published 15 new research papers and 5 editorials as well as numerous presentatinos given at conferences and universities around the world.
We are excited to see what 2013 has in store!
For a full summary of our research activities in 2012 click the link below or any of the links in the sidebar of our publications home page for a reveiw of any year since 2004.
2013 Kamat Group Calendar
Click the link below to download the 2013 Kamat group calendar!

Discovery News - Tandem-Layered Quantum Dot Solar Cells

Discovery News has a recent report on Dr. Pralay Santra and Dr. Prashant Kamat's latest paper showing the synergistic effects seen in layered QDSCs utilizing two and three different colors (compositions) of CdSeS QDs. Click the link below for the full article!
(News.Discovery.com)—"Solar power is becoming more common, but solar cells still aren't great at harvesting all of the available spectrum of sunlight and turning it into electricity, and they can be expensive. So researchers are continually looking for ways to improve the efficiency of solar cells and find better ways to make solar cells cheaply.
"Among them are post doctoral research student Pralay Santra and professor Prashant Kamat, of the University of Notre Dame, who are building solar cells with layers of quantum dots, instead of silicon, which is typically used. Quantum dots are nanometer-sized crystals that glow when stimulated by an external source such as ultraviolet light. They are simpler and cheaper to turn into solar cells than silicon because quantum dots don't require an expensive chip manufacturing plant. Dots can also be tuned to generate electrons when hit with specific wavelengths of light, ranging across the whole spectrum. Silicon cells only respond to reddish and near-infrared light waves.
"'In typical photovoltaics blue light has more energy (than red light) which gets converted to heat,' Kamat told Discovery News. That heat is useless for generating power.
"Kamat and Santra wanted to improve the efficiency of quantum dots and make them more competitive with silicon. A solar cell that uses light from several wavelengths would do that...."
Figure courtesy of Discovery News.
Phys.org - Tandem-Layered Quantum Dot Solar Cells
Phys.org has reported on Pralay Santra and Prashant Kamat's latest paper on the synergistic effects of layered QDSCs utilizing two and three different colors of CdSeS QDs. Click the link below for the full article!
(Phys.org)—"Scientists have discovered that a solar cell consisting of two or three layers of quantum dots, with each layer tuned to a different part of the solar spectrum, has an efficiency that is 40-60% higher than the sum of the efficiencies of separate solar cells each made of one of the individual layers. The synergistic effect of the layered architecture could lead to new ways of designing quantum dot solar cells with high efficiencies and broad-spectrum absorption.
"The researchers who designed and fabricated the new solar cells, Pralay K. Santra and Professor Prashant V. Kamat at the University of Notre Dame in Indiana, wanted to test the previously proposed concept of a rainbow solar cell, which can harvest photons of all "colors" or wavelengths of the visible spectrum.
"In order to fabricate a rainbow solar cell, scientists need to incorporate light-harvesting components with various properties to capture different parts of the spectrum. One way to do this is by using quantum dots, which can be tuned to capture specific wavelengths of light. The typical way of tuning a quantum dot's band gap to capture a particular wavelength of light is to control the dot's size during synthesis...."
Tandem-Layered Quantum Dot Solar Cells
Read the latest paper from the Kamat Lab!
Tandem-Layered Quantum Dot Solar Cells: Tuning the Photovoltaic Response with Luminescent Ternary Cadmium Chalcogenides

Abstract: Photon management in solar cells is an important criterion as it enables the capture of incident visible and infrared photons in an efficient way. Highly luminescent CdSeS quantum dots (QDs) with a diameter of 4.5 nm were prepared with a gradient structure that allows tuning of absorption and emission bands over the entire visible region without varying the particle size. These crystalline ternary cadmium chalcogenides were deposited within a mesoscopic TiO2 film by electrophoretic deposition with a sequentially-layered architecture. This approach enabled us to design tandem layers of CdSeS QDs of varying band gap within the photoactive anode of a QD solar cell (QDSC). An increase in power conversion efficiency of 1.97–2.81% with decreasing band gap was observed for single-layer CdSeS, thus indicating varying degrees of photon harvesting. In two- and three-layered tandem QDSCs, we observed maximum power conversion efficiencies of 3.2 and 3.0%, respectively. These efficiencies are greater than the values obtained for the three individually layered photoanodes. The synergy of using tandem layers of the ternary semiconductor CdSeS in QDSCs was systematically evaluated using transient spectroscopy and photoelectrochemistry.
Galvanic Exchange on RGO
Read the latest paper from the Kamat Lab!
Galvanic Exchange on Reduced Graphene Oxide. Designing a Multifunctional Two‐Dimensional Catalyst Assembly
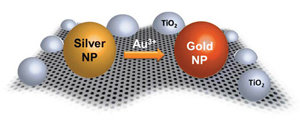
Abstract: The two-dimensional network of reduced graphene oxide (RGO) is decorated with silver and gold nanoparticles. The silver nanoparticles deposited on RGO by photocatalytic reduction are subjected to galvanic exchange with Au3+ ions to transform them into gold nanoparticles. This compositional change on the RGO surface demonstrates RGO's versatile ability to anchor a wide array of nano-particles and facilitate chemical transformations. Coupled with RGO's unique ability to capture and transport electrons, galvanic exchange is used to contrive a two-dimensional nano catalyst mat. Raman studies show that metal nanoparticles anchored on reduced graphene oxide facilitate enhancement of Raman bands. Using methyl viologen as a probe we elucidate the photocatalytic activity of the Semiconductor-RGO-Metal nanoassembly and highlight the mediation of RGO in charge transfer processes.
Graphitic Design
Read the latest paper from the Kamat Lab!
Graphitic Design: Prospects of Graphene-Based Nanocomposites for Solar Energy Conversion, Storage, and Sensing
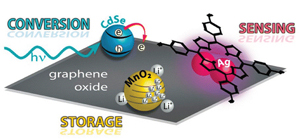
Abstract: Graphene not only possesses interesting electrochemical behavior but also has a remarkable surface area and mechanical strength and is naturally abundant, all advantageous properties for the design of tailored composite materials. Graphene–semiconductor or −metal nanoparticle composites have the potential to function as efficient, multifunctional materials for energy conversion and storage. These next-generation composite systems could possess the capability to integrate conversion and storage of solar energy, detection, and selective destruction of trace environmental contaminants or achieve single-substrate, multistep heterogeneous catalysis. These advanced materials may soon become a reality, based on encouraging results in the key areas of energy conversion and sensing using graphene oxide as a support structure. Through recent advances, chemists can now integrate such processes on a single substrate while using synthetic designs that combine simplicity with a high degree of structural and composition selectivity. This progress represents the beginning of a transformative movement leveraging the advancements of single-purpose chemistry toward the creation of composites designed to address whole-process applications.
The promising field of graphene nanocomposites for sensing and energy applications is based on fundamental studies that explain the electronic interactions between semiconductor or metal nanoparticles and graphene. In particular, reduced graphene oxide is a suitable composite substrate because of its two-dimensional structure, outstanding surface area, and electrical conductivity. In this Account, we describe common assembly methods for graphene composite materials and examine key studies that characterize its excited state interactions. We also discuss strategies to develop graphene composites and control electron capture and transport through the 2D carbon network. In addition, we provide a brief overview of advances in sensing, energy conversion, and storage applications that incorporate graphene-based composites. With these results in mind, we can envision a new class of semiconductor– or metal–graphene composites sensibly tailored to address the pressing need for advanced energy conversion and storage devices.
Charging and Discharging of ZnO NPs on GO
Read the latest paper from the Kamat Lab!
Photoinduced Charging and Discharging of ZnO Nanoparticles on Graphene Oxide Sheets
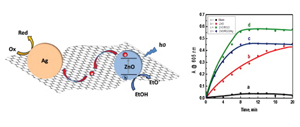
Abstract: Graphene oxide (GO) serves as a two-dimensional carbon nano-mat to anchor catalyst nanoparticles. We have developed a photocatalyst assembly by anchoring ZnO and Ag nanoparticles on graphene oxide sheets suspended in ethanol. Upon photoirradiation, the electrons are transferred from ZnO to GO to produce reduced graphene oxide (RGO). The ZnO–RGO composites are further decorated with Ag nanoparticles by reducing Ag+ ions quantitatively with excess electrons stored in RGO. Under continuous UV-illumination we observe charging of ZnO nanoparticles as evidenced by the shift in absorption edge. However, no shift in the band edge is seen for ZnO–RGO or ZnO–RGO–Ag composites under UV irradiation indicating the quick discharge of electrons on RGO surface. Such charge–discharge phenomenon on the graphene oxide sheet was further probed by carrying out reduction of methyl viologen. Improved charge separation and selectivity in the reduction process was achieved in these graphene based photocatalytic assemblies.
Synthetic Secrets - Fluorescent Ag8 Clusters
Interested in synthesizing fluorescent silver clusters?
Fluorescent Ag8 clusters were recently employed by Wei-Ta Chen in his paper Realizing Visible Photoactivity of Metal Nanoparticles: Excited-State Behavior and Electron-Transfer Properties of Silver (Ag8) Clusters. Find the detailed synthesis procedure by either clicking the link below or visiting our Synthetic Secrets page located under the Resources tab on our website. The synthetic secrets page is a great way to access detailed, step-by-step procedures for a varitey of different matterials - Check it out!
Visible Photoactivity of Silver Clusters
Read the latest paper from the Kamat Lab!
Realizing Visible Photoactivity of Metal Nanoparticles: Excited-State Behavior and Electron-Transfer Properties of Silver (Ag8) Clusters
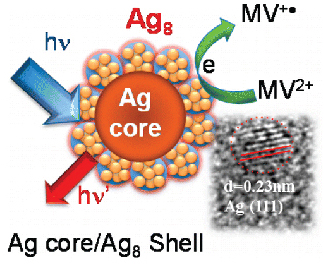
Abstract: Silver nanoclusters complexed with dihydrolipoic acid (DHLA) exhibit molecular-like excited-state properties with well-defined absorption and emission features. The 1.8 nm diameter Ag nanoparticles capped with Ag8 clusters exhibit fluorescence maximum at 660 nm with a quantum yield of 0.07%. Although the excited state is relatively short-lived (τ 130 ps), it exhibits significant photochemical reactivity. By introducing MV2+ as a probe, we have succeeded in elucidating the interfacial electron transfer dynamics of Ag nanoclusters. The formation of MV+• as the electron-transfer product with a rate constant of 2.74 × 1010 s–1 confirms the ability of these metal clusters to participate in the photocatalytic reduction process. Basic understanding of excited-state processes in fluorescent metal clusters paves the way toward the development of biological probes, sensors, and catalysts in energy conversion devices.
A Tough Loss for the Lab Rats

In the final game of the season, the Lab Rats needed a victory to make the playoffs. After a blistering start to the game, the Lab Rats found themselves winning 7-0 after the second inning. The MBAs finally scored 3 runs in the 3rd inning, but the Lab Rats continued to dominate, scoring 4 more runs in the 3rd despite a stupid call to end the inning. With two outs and two men on base, Coach Joe Manser stepped up to the plate and smoked one far over the heads of the outfielders. After all three runners jogged easily around the bases, the first baseman yelled that Coach Manser had not stepped on 1st base (he stepped right next to it so the base wouldn't slip on the grass and make him fall since it was just sitting on top of the grass). The MBAs threw the ball to 1st and, instead of realilzing that this type of play would definitely be "bush league" and simply conceding that he easily scored, procceded to call Coach out. Despite the score stil being 11-3 after the 3rd, these three runs would come back to haunt the Lab Rats. After this conterversial call by the MBAs, the Lab Rats could never quite recover and ended the game losing 13-11.
The Kamat Lab - Summer 2012
Our group has been rapidly expanding in the last few months! We now have students joining us from India, Chile, and the US, Chemistry teachers from schools around South Bend, as well as graduate students, postdocs, undergraduates, and a high school student! We welcome all of our new group members - now let's do some research!
Reduced Graphene Oxide-Pt Composite
Read the latest paper from the Kamat Lab!
Dual-frequency ultrasound for designing two dimensional catalyst surface: Reduced graphene oxide–Pt composite
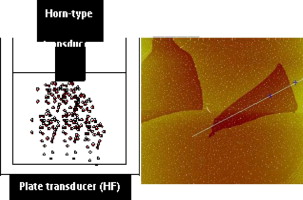
Abstract: Few-layered reduced graphene oxide–Pt composites are prepared using a combination of two ultrasound frequencies at 20 kHz and 211 kHz. Such a unique dual frequency arrangement operating in tandem, yields large exfoliated graphene sheets with platinum nanoparticles dispersed on them. The extent of reduction achieved by the use of this dual frequency sonication arrangement is evaluated by XPS, IR, and Raman spectroscopies. Transmission electron and atomic force microscopies confirm the morphology of resulting assemblies to be bi- and single layered sheets. These composites show good electrocatalytic activity towards methanol oxidation.
Lab Rats Win Despite Losing

The second softball game of the year was technically a victory for the Lab Rats. The Honey Badgers showed up to the game with only 7 players so they lost in a forfeit, but then we decided to play a friendly scrimmage. We could never quite keep up with the Honey Badger's hitting, despite Dr. Kamat and Sean Murphy joining us in the 3rd inning. Dr. Kamat and Sean each had 2 hits in their 2 at bats, but it didn't make up for the rest of us. When we stepped off the field at the end of the game, the final score was Lab Rats 6, Honey Badgers 20.
There was marked improvement on the defensive end for the Lab Rats and we had some great catches in the outfield by Pam, but we need a little (a lot) more practice batting if we are going to win the next game and make it to the playoffs.
Follow the link below to see some pictures of the game on our Facebook page
Webinar - Graphene Oxide Films for SERRS Sensing
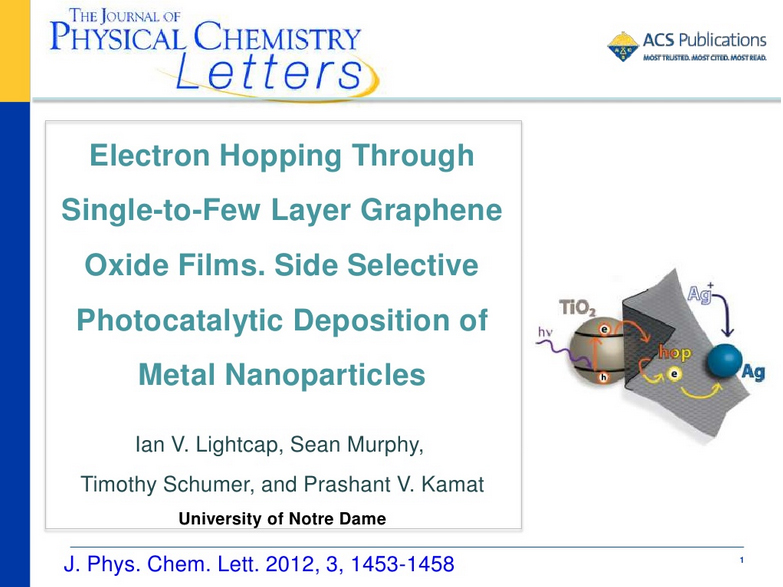
View a Slideshare webinar (slides with audio) of one of our groups most recent papers entitled:
Electron Hopping Through Single-to-Few-Layer Graphene Oxide Films. Side-Selective Photocatalytic Deposition of Metal Nanoparticles
Abstract:Single- to few-layer graphene oxide (GO) sheets have been successfully anchored onto TiO2 films using electrophoretic deposition. Upon UV illumination of TiO2–GO films, photogenerated electrons from TiO2 are captured by GO. These electrons are initially used in GO's reduction, while additional electron transfer results in storage across its sp2 network. In the presence of silver ions, deposition of silver nanoparticles (NPs) is accomplished on the GO surface opposite the TiO2, thus confirming the ability of GO to transport electrons through its plane. Illumination-controlled reduction of silver ions allows for simple selection of particle size and loading, making these semiconductor–graphene–metal (SGM) films ideal for custom catalysis and sensor applications. Initial testing of SGM films as surface-enhanced resonance Raman (SERRS) sensors produced significant target molecule signal enhancements, enabling detection of nanomolar concentrations. Full Paper
The Rad Lab Lab Rats Fall 21-9

After a promising first inning with several nice hits and two homeruns - by Jeff Christians and Coach Joe Manser - the Rad Lab Lab Rats broke out to an early 4-0 lead over the Socio Slammers. The Slammers turned up the defense and got their bats going to fight back and take the lead from the Lab Rats in the 5th. With the score 9-6 for the Slammers, the Lab Rats took the field in the top of the 7th. In this final inning the Slammers really got their bats going. With the help of numerous costly errors in the field by the Lab Rats, the Slammers drove in 12 runs in the inning, putting the Lab Rats down 21-6. In their final at bat, the Lab Rats had some nice hits but just couldn't overcome the 15 run deficit, finishing the game 21-9.
Follow the link below to see some pictures of the game on our Facebook page
Rad Lab Lab Rats Softball Game!
Tomorrow at 5:30pm is the first game of the season for the Rad Lab Lab Rats softball team! Our team shirts are in, we're all practiced up, and hopefully Joe Manser can hit a lot of home runs, because the rest of us are really, really bad :)
If you're around Notre Dame's campus at all tomorrow drop by the fields by the Stepan Center and cheer us on, or grab a glove and join us! It should be a lot of fun but bring water - it is supposed to get up to 100°F!
Check back on Friday for an update on the game.
-Ian Lighcap - Rohm and Haas Outstanding Graduate Student Award

Join us in congratulating Ian Lightcap on winning the Rohm & Haas Outstanding Graduate Student Award from the Department of Chemistry and Biochemistry. Congratulations Ian!
Synchronized Energy and Electron Transfer
Read the latest paper from the Kamat Lab!
Synchronized Energy and Electron Transfer Processes in Covalently Linked CdSe–Squaraine Dye–TiO2 Light Harvesting Assembly
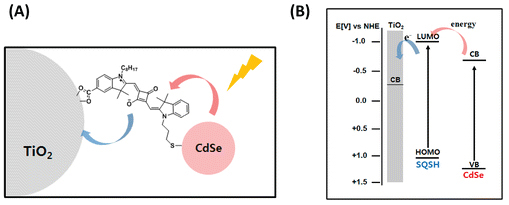
Abstract: Manipulation of energy and electron transfer processes in a light harvesting assembly is an important criterion to mimic natural photosynthesis. We have now succeeded in sequentially assembling CdSe quantum dot (QD) and squaraine dye (SQSH) on TiO2 film and couple energy and electron transfer processes to generate photocurrent in a hybrid solar cell. When attached separately, both CdSe QDs and SQSH inject electrons into TiO2 under visible–near-IR irradiation. However, CdSe QD if linked to TiO2 with SQSH linker participates in an energy transfer process. The hybrid solar cells prepared with squaraine dye as a linker between CdSe QD and TiO2 exhibited power conversion efficiency of 3.65% and good stability during illumination with global AM 1.5 solar condition. Transient absorption spectroscopy measurements provided further insight into the energy transfer between excited CdSe QD and SQSH (rate constant of 6.7 × 1010 s–1) and interfacial electron transfer between excited SQSH and TiO2 (rate constant of 1.2 × 1011 s–1). The synergy of covalently linked semiconductor quantum dots and near-IR absorbing squaraine dye provides new opportunities to harvest photons from selective regions of the solar spectrum in an efficient manner.
Chemical Sensors
Development of chemical sensors is of interest for detecting a vast array of targets such as chemical warfare agents, environmental contaminants, and explosive materials. High sensitivity as well as selectivity are ideal characteristics for a sensor material. Surface enhanced Raman scattering (SERS) active materials are capable of sensing molecules at very low concentration. A current research aim of our lab is to develop sensors capable of both detection of trace contaminants as well as degradation of the contaminants via photocatalytic processes.
Read more about our research on chemical sensors on our Chemical Sensors research page.
QD Oxidation Effects
Read the latest paper from the Kamat Lab!
Photoinduced Surface Oxidation and Its Effect on the Exciton Dynamics of CdSe Quantum Dots
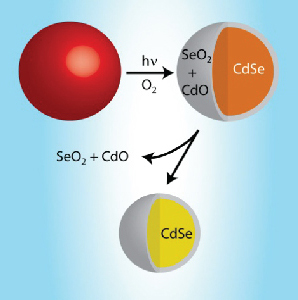
Abstract: With increased interest in semiconductor nanoparticles for use in quantum dot solar cells, there comes a need to understand the long term photo-stability of such materials. Colloidal CdSe quantum dots (QDs) were suspended in toluene and stored in combinations of light/dark and N2/O2 to simulate four possible bench-top storage environments. CdSe QDs stored in a dark, oxygen-free environment were observed to better retain their optical properties over the course of 90 days. The excited state lifetimes as, determined through femtosecond transient absorption spectroscopy, of air-equilibrated samples exposed to light exhibit a decrease in average lifetime (0.81 ns) as compared to samples stored in a nitrogen/dark environment (8.3 ns). A photo-etching technique commonly used for controlled reduction of QD size was found to induce energetic trap states into CdSe QDs and accelerate the rate of electron-hole recombination. X-Ray Absorption Near Edge Structure (XANES) analysis confirms surface oxidation, the extent of which is shown to be dependent on the thickness of the ligand shell.
Graphene Chemistry
In recent years, much of our, and other groups around the globe, research has centered around an exciting new material - Graphene! This material has very diverse potential applications in nano-electronics, batteries, fuel cells, solar cells, and much more!
Our group's research page, describing some of our latest research utilizing graphene and graphene composites for improved solar cells, lithium ion batteries, and customizable conductive mats for sensing and catalysis, is updated and now online. Check out some of our latest work on graphene chemistry!
2012 Graduation!

Congratulations to Dr. Matt Becker, Dr. Ben Meekins, and Dr. Ian Lightcap, the newest doctors to come out of the Kamat lab!
Matt Becker is now working as a teaching professor at the Roechester Institute of Technology in New York, teaching introductory Physics, Ben Meekins is going to be working as a postdoc in Allen Bard's group at the University of Texas in Austin, Texas, and Ian Lightcap is staying at the University of Notre Dame, directing the cSEND Transformative Solar Facility.
Semiconductor-Graphene-Metal Films in PhysOrg
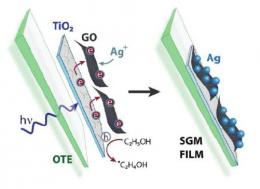
Graphene has many promising applications on its own, but pairing the two-dimensional material with the semiconductor titanium dioxide (TiO2) extends its capabilities even further. A team of chemists at the University of Notre Dame in Notre Dame, Indiana, has demonstrated that graphene oxide (GO)-TiO2 films, when illuminated, cause electrons to hop from one side of the film to the other. When adding silver ions to the picture, this electron hopping can create films that have a semiconductor on one side of the GO and metal on the other. The resulting semiconductor-graphene-metal (SGM) films could serve as highly sensitive chemical sensors.
Follow the link to read more of the PhysOrg article!
Electron Hopping Through GO Films
Read the latest paper from the Kamat Lab!
Electron Hopping Through Single-to-Few-Layer Graphene Oxide Films. Side-Selective Photocatalytic Deposition of Metal Nanoparticles
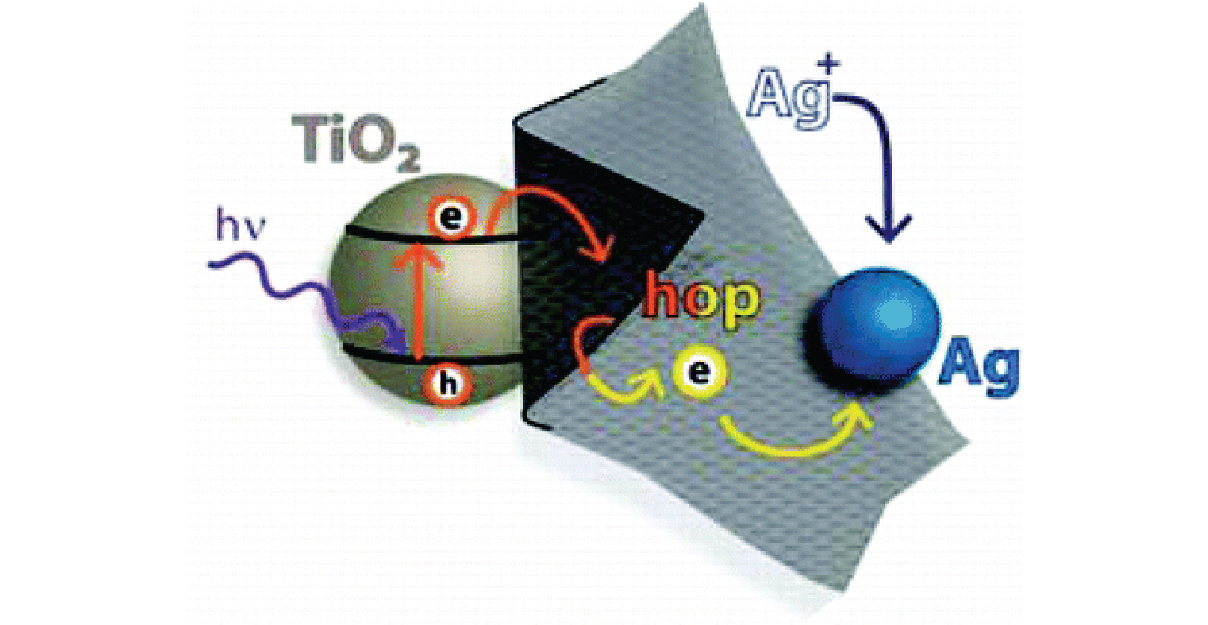
Abstract: Single- to few-layer graphene oxide (GO) sheets have been successfully anchored onto TiO2 films using electrophoretic deposition. Upon UV illumination of TiO2–GO films, photogenerated electrons from TiO2 are captured by GO. These electrons are initially used in GO's reduction, while additional electron transfer results in storage across its sp2 network. In the presence of silver ions, deposition of silver nanoparticles (NPs) is accomplished on the GO surface opposite the TiO2, thus confirming the ability of GO to transport electrons through its plane. Illumination-controlled reduction of silver ions allows for simple selection of particle size and loading, making these semiconductor–graphene–metal (SGM) films ideal for custom catalysis and sensor applications. Initial testing of SGM films as surface-enhanced resonance Raman (SERRS) sensors produced significant target molecule signal enhancements, enabling detection of nanomolar concentrations.
Solar Energy - Beyond the Hype

Professor Prashant Kamat goes beyond the narrow focus of research and extends the discussion out to the big picture of energy and the environment. He gives some of the basic facts about how we create and use energy around the world today and how this is expected to change in the future, offering up the 10 terawatt challenge - that 10 TW of carbon neutral energy will be needed to avert dangerous antropogenic climate change. Then some possible carbon neutral ways to supply our growing energy demand and produce the desired 10 TW of carbon neutral energy. Dr. Kamat finally shows why he believes that solar energy is the only source which can adaquately meet this growing energy demand and a few of the different approaches that researchers in our lab are taking to help make widespread solar energy a reality.
Boosting Quantum Dot Solar Cell Efficiency
Read the latest paper from the Kamat Lab!
Boosting the Efficiency of Quantum Dot Sensitized Solar Cells Through Modulation of Interfacial Charge Transfer
Abstract: The demand for clean energy will require the design of nanostructure-based light-harvesting assemblies for the conversion of solar energy into chemical energy (solar fuels) and electrical energy (solar cells). Semiconductor nanocrystals serve as the building blocks for designing next generation solar cells, and metal chalcogenides (e.g., CdS, CdSe, PbS, and PbSe) are particularly useful for harnessing size-dependent optical and electronic properties in these nanostructures.
This Account focuses on photoinduced electron transfer processes in quantum dot sensitized solar cells (QDSCs) and discusses strategies to overcome the limitations of various interfacial electron transfer processes. The heterojunction of two semiconductor nanocrystals with matched band energies (e.g., TiO2 and CdSe) facilitates charge separation. The rate at which these separated charge carriers are driven toward opposing electrodes is a major factor that dictates the overall photocurrent generation efficiency. The hole transfer at the semiconductor remains a major bottleneck in QDSCs. For example, the rate constant for hole transfer is 2–3 orders of magnitude lower than the electron injection from excited CdSe into oxide (e.g., TiO2) semiconductor. Disparity between the electron and hole scavenging rate leads to further accumulation of holes within the CdSe QD and increases the rate of electron–hole recombination. To overcome the losses due to charge recombination processes at the interface, researchers need to accelerate electron and hole transport.
The power conversion efficiency for liquid junction and solid state quantum dot solar cells, which is in the range of 5–6%, represents a significant advance toward effective utilization of nanomaterials for solar cells. The design of new semiconductor architectures could address many of the issues related to modulation of various charge transfer steps. With the resolution of those problems, the efficiencies of QDSCs could approach those of dye sensitized solar cells (DSSC) and organic photovoltaics.
Prashant Kamat - ND Thinks Big

In March, the University of Notre Dame held a lecture series entitled, ND Thinks Big. This series - modeled after the popular TED talks, "Ideas worth sharing" - featured short lectures by 10 of Notre Dame's most exciting and engaging professors for a variety of disciplines.
One of the professors invited to give a talk at this event was Dr. Prashant Kamat! You see the talk Dr. Kamat gave at this event on sustainability and energy on YouTube by following the link below.
Project Infinite Green

Last week Friday, Project Infinite Green visited our lab to learn more about solar energy!
Project Infinite Green is an after-school group started in 2011 offered through Lemont High School District in Illinois to 7th and 8th grade students in the area. Thier goal is to educate these students on many of the energy issues we face today, and examine how energy useage effects the world we live in.
You can view pictures of their visit on the Kamat Lab's Facebook page.
To learn more about Project Infinite Green by following the link below or visiting Project Infinite Green on Facebook!
Know Thy Nano Neighbor
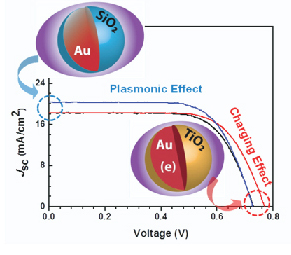
Read the latest paper from the Kamat Lab!
Know Thy Nano Neighbor. Plasmonic versus Electron Charging Effects of Metal Nanoparticles in Dye Sensitized Solar Cells.
Abstract: Neighboring metal nanoparticles influence photovoltaic and photocatalytic behavior of semiconductor nanostructures either through Fermi level equilibration by accepting electrons or inducing localized surface plasmon effects. By employing SiO2 and TiO2 capped Au nanoparticles we have identified the mechanism with which the performance of dye sensitized solar cells (DSSC) is influenced by a neighboring metal nanoparticles. The efficiency of N719 dye sensitized solar cell, (9.3%) increased to 10.2% upon incorporation of 0.7% of Au@SiO2 and 9.8% upon loading of 0.7% of Au@TiO2 nanoparticles. The plasmonic effect as monitored by introducing Au@SiO2 in DSSC produces higher photocurrent. However, Au nanoparticles undergo charge equilibration with TiO2 nanoparticles and shift the Fermi level of the composite to more negative potentials. As a result Au@TiO2 nanoparticles embedded DSSC exhibit higher photovoltage. A better understanding of these two effects is crucial in exploiting the beneficial aspects of metal nanoparticles in photovoltaics.
Solar Hydrogen Generation
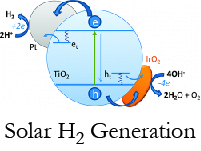
Using sunlight to efficiently split water into hydrogen and oxygen is an exciting approach to storage of solar energy in a chemical form. Our lab has recently done a variety of work with the aim of making this an industrially viable process: demonstrating a system for continuous hydrogen generation, and revealing the role of the water oxidation catalyst, IrO2, in a photocatalytic system.
If you are interested in learning more about Hydrogen Generation, follow the link to our research page.
Ian Lightcap joins cSEND

Ian Lightcap, one of the members of our lab who defended his Ph. D. thesis earlier this month, will be joining the Center for Sustainable Energy at Notre Dame (cSEND). He will be directing the Transformative Solar Facility and performing his own solar fuels research.
Congratulations to Ian and his family on the great job!
Measuring Performance of QDSCs Webinar
View our latest Slideshare webinar (slides with audio) entitled:
Measuring Photoelectrochemical Performance of QDSCs Interpreting 2 and 3 Electrode Measurements
This slideshare presentation focuses on some of the background information necessary in understanding photoelectrochemical measurements, and then covers some of the basics of 2 and 3 electrode characterization.
Dr. Ian Lightcap

Congratulations to Dr. Ian Lightcap on successful completion of his Ph. D. in Chemistry! Ian successfully defended his doctoral thesis entitled - Excited State Interactions in Graphene Oxide-Semiconductor/Metal Nanoparticle Architectures for Sensing and Energy Conversion. Congratulations on completion of your Ph. D. Ian!
CdSe/GO for Light Energy Conversion
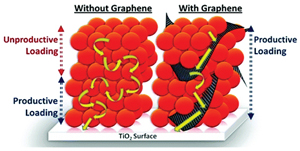
Read the latest paper from the Kamat Lab!
Fortification of CdSe Quantum Dots with Graphene Oxide. Excited State Interactions and Light Energy Conversion
Abstract: Graphene based 2-D carbon nanostructures provide new opportunities to fortify semiconductor based light harvesting assemblies. Electron and energy transfer rates from photoexcited CdSe colloidal quantum dots (QDs) to graphene oxide (GO) and reduced graphene oxide (RGO) were isolated by analysis of excited state deactivation lifetimes as a function of degree of oxidation and charging in (R)GO. Apparent rate constants for energy and electron transfer determined for CdSe–GO composites were 5.5 × 108 and 6.7 × 108 s–1, respectively. Additionally, incorporation of GO in colloidal CdSe QD films deposited on conducting glass electrodes was found to enhance the charge separation and electron conduction through the QD film, thus allowing three-dimensional sensitization. Photoanodes assembled from CdSe–graphene composites in quantum dot sensitized solar cells display improved photocurrent response (150%) over those prepared without GO.
Dr. Ben Meekins

Join us in congratulating Ben Meekins on successful completion of his Ph. D. in Chemical and Biomolecular Engineering! Ben successfully defended his doctoral thesis entitled - Controlling Interfacial Transfer Processes for Improved Photoelectrochemical Performance. Ben, his wife Maggie, and their three dogs, will soon be leaving for the University of Texas where Ben will continue his research as a post doctoral researcher in Allen Bard's group. Congratulations Ben!
Origin of RGO Enhancements Webinar
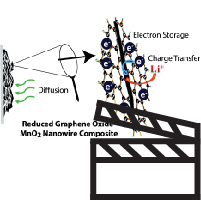
View a Slideshare webinar (slides with audio) of one of our groups most recent papers entitled:
Origin of Reduced Graphene Oxide Enhancements in Electrochemical Energy Storage
Abstract: Reduced graphene oxide (RGO) has become a common substrate upon which active intercalation materials are anchored for electrochemical applications such as supercapacitors and lithium ion batteries. The unique attributes of RGO, including high conductivity and porous macrostructure, are often credited for enhanced cycling and capacity performance. Here we focus on probing the electrochemical response of α-MnO2/RGO composite used as an electrode in a lithium ion battery cell and elucidating the mechanistic aspects of the RGO on the commonly observed improvements in cycling and capacity. We find that electron storage properties of RGO enables better electrode kinetics, more rapid diffusion of Li+ to intercalation sites, and a greater capacitance effect during discharge. Further investigation of the length of the one-dimensional nanowire morphology of the α-MnO2 has allowed us to differentiate between the innate characteristics of the MnO2 and those of the RGO. RGO coupled with long nanowires (>5 μm) exhibited the best performance in all tests and retained 150 mAh/g capacity after 20 cycles at 0.4C rate. Full Paper
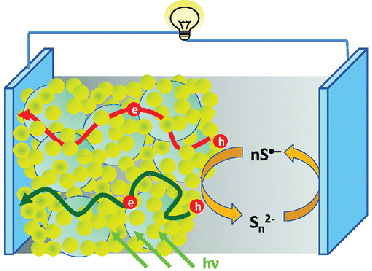
Cu2S/RGO Counter Electrode Webinar
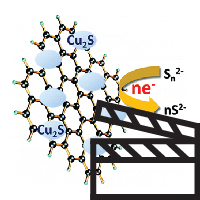
View a Slideshare webinar (slides with audio) of one of our groups most recent papers entitled:
Cu2S Reduced Graphene Oxide Composite for High-Efficiency Quantum Dot Solar Cells. Overcoming the Redox Limitations of S2–/Sn2– at the Counter Electrode
Abstract: Polysulfide electrolyte that is employed as a redox electrolyte in quantum dot sensitized solar cells provides stability to the cadmium chalcogenide photoanode but introduces significant redox limitations at the counter electrode through undesirable surface reactions. By designing reduced graphene oxide (RGO)-Cu2S composite, we have now succeeded in shuttling electrons through the RGO sheets and polysulfide-active Cu2S more efficiently than Pt electrode, improving the fill factor by 75%. The composite material characterized and optimized at different compositions indicates a Cu/RGO mass ratio of 4 provides the best electrochemical performance. A sandwich CdSe quantum dot sensitized solar cell constructed using the optimized RGO-Cu2S composite counter electrode exhibited an unsurpassed power conversion efficiency of 4.4%. Full Paper
KamatLab.com and the Latest Updates

Visiting the Prashant Kamat Laboratory is now easier than ever! Simply type KamatLab.com into any web browser and you will instantly be redirected to our home page. You no longer have to search for our website or remember a complicated URL!
Over the next weeks we are also making a concerted effort to update the Research Pages with information about our different areas of research, Synthetic Secrets with detailed procedures for making a variety of materials and devices, and Facilities page with information about all the equipment and instruments available in the Radiation Laboratory and at the University of Notre Dame. Check back often for the latest updates!
ND Thinks Big: 10 Speakers, 10 Minutes, 10 Big Ideas.

Sponsored by The Hub and CUSE, ND Thinks Big will feature 10 of Notre Dame's most exciting and engaging professors sharing the impact of their work in action-packed, accessible 10 minute talks.
Modeled after the mission of TED talks, "Ideas worth sharing", ND Thinks Big will be a dynamic and energetic event with talks from faculty in all of Notre Dame's colleges.
Dr. Kamat will be one of the spaekers, sharing some ideas from our research on sustainability and solar energy.
Solar Paint in C&EN

To fabricate solar cells, engineers may someday trade their clean rooms for a paint smock. Researchers have developed a paste of semiconducting nanoparticles called solar paint that could lead to cheaper and easier-to-produce solar cells .
Many researchers working on solar energy have focused on improving the efficiency of silicon-based solar cells. But silicon devices have a high price tag because of the specialized protocols and equipment needed, says Prashant Kamat of the University of Notre Dame. He points out that some researchers have avoided using silicon and created cells using quantum dots made from materials like lead sulfide. Unfortunately, the fabrication process for these quantum dot solar cells is still expensive and slow. To simplify and lower the costs of solar cell production, Kamat and his colleagues wanted to develop quantum dot materials that someone could paint onto any conductive surface without needing special equipment.
Top 100 Chemists, 2000-2010
In February, 2011, Dr. Kamat was named to Thompson Reuters list of the top 100 chemists of the past 11 years. Dr. Kamat ranked number 58 on the list which was generated based on the citation impact score for papers published in the field.
Prashant Named AAAS Fellow

In December 2010, the AAAS Council (American Association for the Advancement of Science) elected 503 members as Fellows of AAAS. Dr. Prashant Kamat has been named one of these fellows, a distinction recognizing his contributions to science and technology.
