585. From Light to Dark: Dancing with Electrons in Colloidal 2D MoS2 Nanosheets Bo-An Chen, Nathaniel L. Dominique, Anthony Kipkorir, Jon P. Camden, Sylwia Ptasinska and Prashant V. Kamat
J. Phys. Chem. Lett. 2024, 15, XXX, 4920-4927.
 Extending the lifetime of photogenerated electrons in semiconductor systems is an important criterion for the conversion of light into storable energy. We have now succeeded in storing electrons in a photoirradiated colloidal molybdenum disulfide (MoS2) suspension, showcasing its unique reversible photoresponsive behavior. The dampened A and B excitonic peaks indicate the accumulation of photogenerated electrons and the minimization of interactions between MoS2 interlayers. The stored electrons were quantitatively extracted by titrating with a ferrocenium ion in the dark, giving ca. 0.2 electrons per MoS2 formula unit. The emergence of the photoinduced A1g* Raman mode and the decrease in zeta potential after irradiation suggest intercalation of counterions to maintain overall charge balance upon electron storage. These results provide insights into the mechanism of photogenerated electron storage in 2D materials and pave the way for the potential application of colloidal 2D materials in electron storage.
Extending the lifetime of photogenerated electrons in semiconductor systems is an important criterion for the conversion of light into storable energy. We have now succeeded in storing electrons in a photoirradiated colloidal molybdenum disulfide (MoS2) suspension, showcasing its unique reversible photoresponsive behavior. The dampened A and B excitonic peaks indicate the accumulation of photogenerated electrons and the minimization of interactions between MoS2 interlayers. The stored electrons were quantitatively extracted by titrating with a ferrocenium ion in the dark, giving ca. 0.2 electrons per MoS2 formula unit. The emergence of the photoinduced A1g* Raman mode and the decrease in zeta potential after irradiation suggest intercalation of counterions to maintain overall charge balance upon electron storage. These results provide insights into the mechanism of photogenerated electron storage in 2D materials and pave the way for the potential application of colloidal 2D materials in electron storage.
584. How Effective Are Sub-Bandgap States in AgInS2 States in AgInS2 Quantum Dots for Electron Transfer? Yiseul Yu, Anthony Kipkorir, Myong Yong Choi, and Prashant V. Kamat
Chem. Mater 2024, XXXX, XXX, XXX-XXX.
 Ternary I–III–VI2 semiconductors, such as CuInS2 and AgInS2 (compliant with RoHS, restriction of hazardous substances), are useful as light-harvesting materials. However, the presence of sub-bandgap states (donor–acceptor pair or DAP) introduces complexity during their activation through photoexcitation. When photoirradiated, the photogenerated charge carriers in AgInS2 quantum dots undergo rapid relaxation to populate intrinsic DAP states while competing with charge carrier recombination. Interestingly, these defect-related DAP states can be activated through sub-bandgap excitation and, thus, extend the absorption range to the near-infrared region. We have now employed time-resolved absorption and emission techniques to glean mechanistic insights into the photophysical properties of intragap states of AgInS2 quantum dots (QDs) and their participation in interfacial electron transfer. When the AgInS2 QDs are excited with above bandgap excitation (400 nm), we observe a prompt formation (< 1 ps) of the bleach at wavelengths closer to the bandgap, indicating the formation of a charge-separated pair. This transient bleach shifts to lower energies with time (∼5 ps), indicating population of sub-bandgap states via relaxation of electrons and holes from the conduction and valence bands, respectively. These sub-bandgap states which can also be populated via direct excitation using low energy (λ < Eg) excitation exhibit prompt bleach (< 1 ps) in contrast to bandgap excitation. The excited DAP states are long-lived (∼1 μs) and can participate in the electron transfer process. We have elucidated the electron transfer dynamics from these midgap states of AgInS2 by employing ethyl viologen (EV2+) as a probe molecule. The role of surface-anchored viologen as an electron shuttle was further exploited by using free-floating benzoquinone (BQ) as a secondary electron acceptor. The sub-bandgap response of AgInS2 to promote electron transfer paves the way to extend the photoresponse of ternary I–III–VI2 semiconductor-based photocatalytic systems.
Ternary I–III–VI2 semiconductors, such as CuInS2 and AgInS2 (compliant with RoHS, restriction of hazardous substances), are useful as light-harvesting materials. However, the presence of sub-bandgap states (donor–acceptor pair or DAP) introduces complexity during their activation through photoexcitation. When photoirradiated, the photogenerated charge carriers in AgInS2 quantum dots undergo rapid relaxation to populate intrinsic DAP states while competing with charge carrier recombination. Interestingly, these defect-related DAP states can be activated through sub-bandgap excitation and, thus, extend the absorption range to the near-infrared region. We have now employed time-resolved absorption and emission techniques to glean mechanistic insights into the photophysical properties of intragap states of AgInS2 quantum dots (QDs) and their participation in interfacial electron transfer. When the AgInS2 QDs are excited with above bandgap excitation (400 nm), we observe a prompt formation (< 1 ps) of the bleach at wavelengths closer to the bandgap, indicating the formation of a charge-separated pair. This transient bleach shifts to lower energies with time (∼5 ps), indicating population of sub-bandgap states via relaxation of electrons and holes from the conduction and valence bands, respectively. These sub-bandgap states which can also be populated via direct excitation using low energy (λ < Eg) excitation exhibit prompt bleach (< 1 ps) in contrast to bandgap excitation. The excited DAP states are long-lived (∼1 μs) and can participate in the electron transfer process. We have elucidated the electron transfer dynamics from these midgap states of AgInS2 by employing ethyl viologen (EV2+) as a probe molecule. The role of surface-anchored viologen as an electron shuttle was further exploited by using free-floating benzoquinone (BQ) as a secondary electron acceptor. The sub-bandgap response of AgInS2 to promote electron transfer paves the way to extend the photoresponse of ternary I–III–VI2 semiconductor-based photocatalytic systems.
583. Photocatalytic Membrane for Hydrogen Evolution: Directed Electron and Hole Transfer across Pt-AgInS2-Nafion Yiseul Yu, Anthony Kipkorir, Myong Yong Choi, and Prashant V. Kamat
ACS Materials Lett. 2024, 6, XXX, 1856-1862.
 A photocatalytically active membrane, designed by embedding AgInS2 semiconductor nanoparticles and a Pt cocatalyst, facilitates a “vectorial” flow of photogenerated electrons and holes in opposite directions. The fabricated Pt–AgInS2–Nafion membrane, when inserted in an H-cell containing 50% ethanol (AgInS2 side) and water (pH = 4, Pt side), produced H2 under visible light irradiation. Photogenerated electrons reduced H+ at the Pt surface to produce H2, while oxidation of ethanol with holes at AgInS2 also produced H2. Back electron transfer at the Pt surface and surface defects within AgInS2 were responsible for the lower H2 yield in the reduction compartment. Remediation of the AgInS2 film with mercaptopropionic acid increased the yield 5–10 times by overcoming the loss of charge carriers at the defect sites. The feasibility of carrying out selective reduction and oxidation processes by directing the flow of charge carriers highlights the usefulness of photocatalytic membranes in solar fuel generation.
A photocatalytically active membrane, designed by embedding AgInS2 semiconductor nanoparticles and a Pt cocatalyst, facilitates a “vectorial” flow of photogenerated electrons and holes in opposite directions. The fabricated Pt–AgInS2–Nafion membrane, when inserted in an H-cell containing 50% ethanol (AgInS2 side) and water (pH = 4, Pt side), produced H2 under visible light irradiation. Photogenerated electrons reduced H+ at the Pt surface to produce H2, while oxidation of ethanol with holes at AgInS2 also produced H2. Back electron transfer at the Pt surface and surface defects within AgInS2 were responsible for the lower H2 yield in the reduction compartment. Remediation of the AgInS2 film with mercaptopropionic acid increased the yield 5–10 times by overcoming the loss of charge carriers at the defect sites. The feasibility of carrying out selective reduction and oxidation processes by directing the flow of charge carriers highlights the usefulness of photocatalytic membranes in solar fuel generation.
582. Energy Cascade in Halide Perovskite-Multiple Chromophore Films: Direct versus Mediated Transfer Jishnudas Chakkamalayath, Lauren E. Martin, and Prashant V. Kamat
ACS Photonics 2024, XXXX, XXX, XXX-XXX.
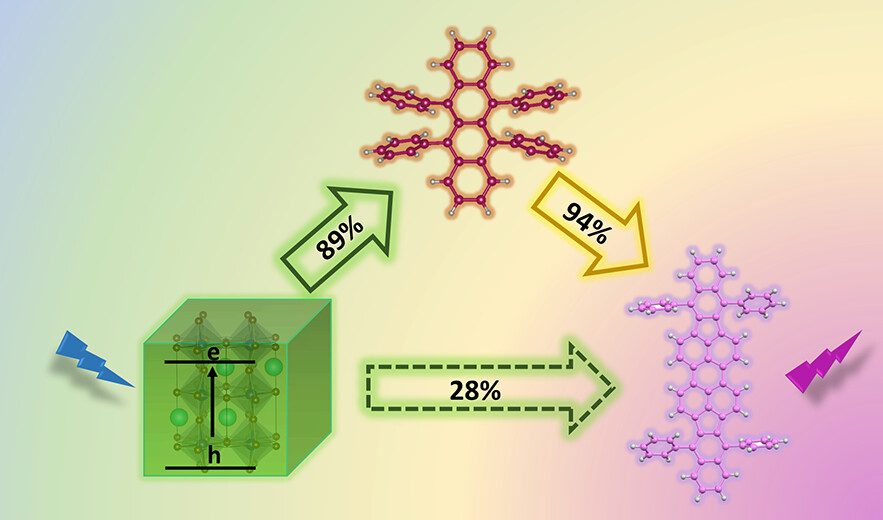 The capability of halide perovskite nanocrystals to sensitize both singlet and triplet excited states of a chromophore highlights their potential as photosensitizers in down conversion (singlet energy transfer) or upconversion (triplet energy transfer followed by triplet–triplet annihilation) applications. In semiconductor-multiple chromophore assemblies, however, various possibilities arise to modulate the energy transfer process and hence the final emission output. By employing CsPbBr3 nanocrystals (Eg = 2.47 eV) as a primary sensitizer, we have now probed the singlet energy transfer to two dyes, viz., rubrene (Es = 2.23 eV) and DBP, a perylene derivative (Es = 2.07 eV). By first characterizing the excited-state interactions between CsPbBr3 and individual dye pairs, we establish the favorable pathway for singlet energy transfer in a donor–acceptor1–acceptor2 assembly. The singlet energy transfer efficiencies for CsPbBr3-rubrene, rubrene-DBP, and CsPbBr3-DBP systems were quantified as 89%, 94%, and 28%, respectively. When all three components (CsPbBr3-rubrene-DBP) are present in the film, we observe a cascading energy transfer to yield a high population of singlet excited-states in DBP. The 1DBP* yield increased with rubrene concentration, thus confirming its mediating role in the energy cascade. Thus, a proper choice of mediator can promote singlet energy transfer when the spectral overlap between a donor and acceptor is poor. Elucidation of the excited-state interactions in CsPbBr3-rubrene DBP films offers new insights into the sensitization of multiple chromophore assemblies and ways to modulate the singlet energy flow.
The capability of halide perovskite nanocrystals to sensitize both singlet and triplet excited states of a chromophore highlights their potential as photosensitizers in down conversion (singlet energy transfer) or upconversion (triplet energy transfer followed by triplet–triplet annihilation) applications. In semiconductor-multiple chromophore assemblies, however, various possibilities arise to modulate the energy transfer process and hence the final emission output. By employing CsPbBr3 nanocrystals (Eg = 2.47 eV) as a primary sensitizer, we have now probed the singlet energy transfer to two dyes, viz., rubrene (Es = 2.23 eV) and DBP, a perylene derivative (Es = 2.07 eV). By first characterizing the excited-state interactions between CsPbBr3 and individual dye pairs, we establish the favorable pathway for singlet energy transfer in a donor–acceptor1–acceptor2 assembly. The singlet energy transfer efficiencies for CsPbBr3-rubrene, rubrene-DBP, and CsPbBr3-DBP systems were quantified as 89%, 94%, and 28%, respectively. When all three components (CsPbBr3-rubrene-DBP) are present in the film, we observe a cascading energy transfer to yield a high population of singlet excited-states in DBP. The 1DBP* yield increased with rubrene concentration, thus confirming its mediating role in the energy cascade. Thus, a proper choice of mediator can promote singlet energy transfer when the spectral overlap between a donor and acceptor is poor. Elucidation of the excited-state interactions in CsPbBr3-rubrene DBP films offers new insights into the sensitization of multiple chromophore assemblies and ways to modulate the singlet energy flow.
581. Ramifications of Ion Migration on 2D Lead Halide Perovskites Preethi Mathew, Junsang Cho, and Prashant V. Kamat
ACS Energy Lett. 2024, 9, 3, 1103-1114.
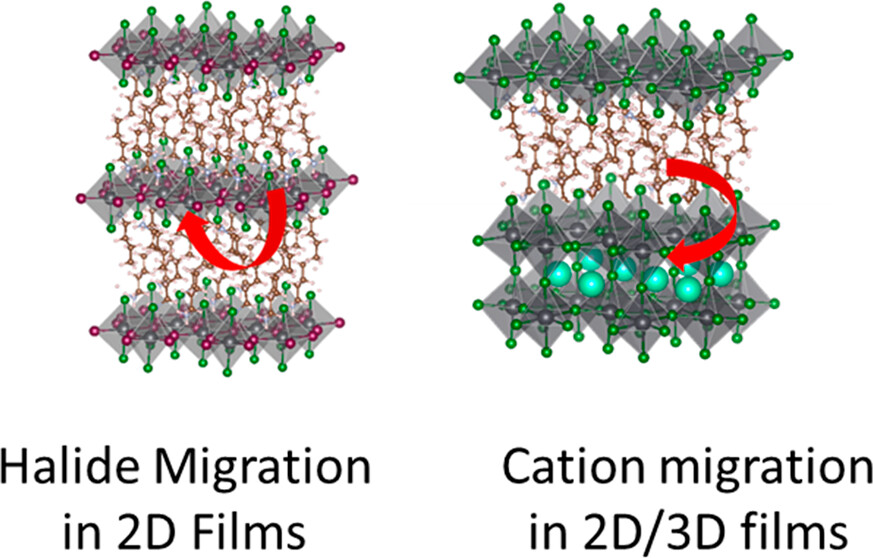 Lower dimensional or 2D halide perovskites, with their versatile structural and functional properties, are known to improve the performance and room temperature stability of perovskite solar cells. One would expect 2D perovskites to be more resistant to ion migration compared to their 3D counterparts because of the presence of bulky organic cations. However, recent findings show that ion migration indeed is prevalent in 2D halide perovskites similar to 3D perovskites. Halide ion migration in 2D perovskites manifests itself in halide ion segregation under photoirradiation as well as in halide exchange between physically paired films of 2D perovskites with different halide ions. Besides halide ion migration, cation migration of spacer cations and A-site cations is also seen when 2D/3D perovskite films are subjected to light and thermal stress. It is important to recognize the importance of ion migration while incorporating 2D perovskites in solar cells and other optoelectronic devices, as it can be detrimental for achieving streamlined performance and long-term stability. This Perspective discusses recent reports on ion migration in 2D and in 2D/3D halide perovskite films under the operational conditions (at elevated temperature and given built-in bias) and presents a few mitigating strategies.
Lower dimensional or 2D halide perovskites, with their versatile structural and functional properties, are known to improve the performance and room temperature stability of perovskite solar cells. One would expect 2D perovskites to be more resistant to ion migration compared to their 3D counterparts because of the presence of bulky organic cations. However, recent findings show that ion migration indeed is prevalent in 2D halide perovskites similar to 3D perovskites. Halide ion migration in 2D perovskites manifests itself in halide ion segregation under photoirradiation as well as in halide exchange between physically paired films of 2D perovskites with different halide ions. Besides halide ion migration, cation migration of spacer cations and A-site cations is also seen when 2D/3D perovskite films are subjected to light and thermal stress. It is important to recognize the importance of ion migration while incorporating 2D perovskites in solar cells and other optoelectronic devices, as it can be detrimental for achieving streamlined performance and long-term stability. This Perspective discusses recent reports on ion migration in 2D and in 2D/3D halide perovskite films under the operational conditions (at elevated temperature and given built-in bias) and presents a few mitigating strategies.
580. Tuning Energy Transfer Pathways in Halide Perovskite–Dye Hybrids through Bandgap Engineering Akshaya Chemmangat, Jishnudas Chakkamalayath, Jeffrey T. DuBose, and Prashant V. Kamat
J. Am. Chem. Soc. 2024, 146, 5, 3352-3362.
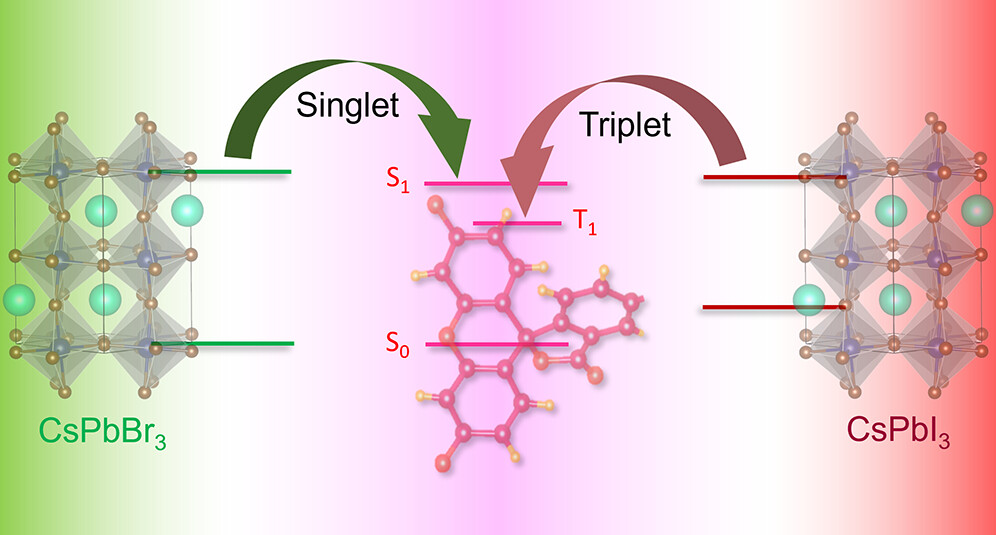 Lead halide perovskite nanocrystals, which offer rich photochemistry, have the potential to capture photons over a wide range of the visible and infrared spectrum for photocatalytic, optoelectronic, and photon conversion applications. Energy transfer from the perovskite nanocrystal to an acceptor dye in the form of a triplet or singlet state offers additional opportunities to tune the properties of the semiconductor–dye hybrid and extend excited-state lifetimes. We have now successfully established the key factors that dictate triplet energy transfer between excited CsPbI3 and surface-bound rhodamine dyes using absorption and emission spectroscopies. The pendant groups on the acceptor dyes influence surface binding to the nanocrystals, which in turn dictate the energy transfer kinetics, as well as the efficiency of energy transfer. Of the three rhodamine dyes investigated (rhodamine B, rhodamine B isothiocyanate, and rose Bengal), the CsPbI3–rose Bengal hybrid with the strongest binding showed the highest triplet energy transfer efficiency (96%) with a rate constant of 1 × 109 s-1. This triplet energy transfer rate constant is nearly 2 orders of magnitude slower than the singlet energy transfer observed for the pure-bromide CsPbBr3–rose Bengal hybrid (1.1 × 1011 s-1). Intriguingly, although the single-halide CsPbBr3 and CsPbI3 nanocrystals selectively populate singlet and triplet excited states of rose Bengal, respectively, the mixed halide perovskites were able to generate a mixture of both singlet and triplet excited states. By tuning the bromide/iodide ratio and thus bandgap energy in CsPb(Br1-xIx)3 compositions, the percentage of singlets vs triplets delivered to the acceptor dye was systematically tuned from 0 to 100%. The excited-state properties of halide perovskite-molecular hybrids discussed here provide new ways to modulate singlet and triplet energy transfer in semiconductor–molecular dye hybrids through acceptor functionalization and donor bandgap engineering.
Lead halide perovskite nanocrystals, which offer rich photochemistry, have the potential to capture photons over a wide range of the visible and infrared spectrum for photocatalytic, optoelectronic, and photon conversion applications. Energy transfer from the perovskite nanocrystal to an acceptor dye in the form of a triplet or singlet state offers additional opportunities to tune the properties of the semiconductor–dye hybrid and extend excited-state lifetimes. We have now successfully established the key factors that dictate triplet energy transfer between excited CsPbI3 and surface-bound rhodamine dyes using absorption and emission spectroscopies. The pendant groups on the acceptor dyes influence surface binding to the nanocrystals, which in turn dictate the energy transfer kinetics, as well as the efficiency of energy transfer. Of the three rhodamine dyes investigated (rhodamine B, rhodamine B isothiocyanate, and rose Bengal), the CsPbI3–rose Bengal hybrid with the strongest binding showed the highest triplet energy transfer efficiency (96%) with a rate constant of 1 × 109 s-1. This triplet energy transfer rate constant is nearly 2 orders of magnitude slower than the singlet energy transfer observed for the pure-bromide CsPbBr3–rose Bengal hybrid (1.1 × 1011 s-1). Intriguingly, although the single-halide CsPbBr3 and CsPbI3 nanocrystals selectively populate singlet and triplet excited states of rose Bengal, respectively, the mixed halide perovskites were able to generate a mixture of both singlet and triplet excited states. By tuning the bromide/iodide ratio and thus bandgap energy in CsPb(Br1-xIx)3 compositions, the percentage of singlets vs triplets delivered to the acceptor dye was systematically tuned from 0 to 100%. The excited-state properties of halide perovskite-molecular hybrids discussed here provide new ways to modulate singlet and triplet energy transfer in semiconductor–molecular dye hybrids through acceptor functionalization and donor bandgap engineering.
579. Directional Electron Transfer across In2S3/ZnS-Embedded Photocatalytic Membranes Yiseul Yu, Anthony Kipkorir, Myong Yong Choi, and Prashant V. Kamat
ACS Appl. Energy Mater. 2024, 7, 2, 681-688.
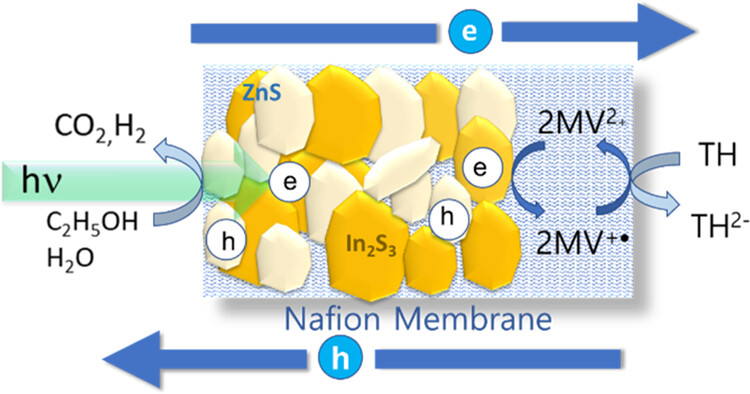 Photocatalytic membranes prepared with semiconductor nanoparticles embedded in a polymer film offer a convenient approach to direct the electron and hole flow and separate reduction and oxidation products. We have now embedded In2S3 and ZnS semiconductor nanoparticles in a Nafion membrane to induce photocatalytic reactions using visible light. In addition, we incorporated a viologen redox relay within the membrane to facilitate electron transfer to thionine (TH) dissolved in water. By inserting the photocatalytic membrane in a H-cell, we can separate the oxidation and reduction products and track the electron flow using steady-state photolysis and transient absorption spectroscopy. The enhanced charge separation in the In2S3 and ZnS heterostructure at 50:50 loading allowed us to maximize the electron-transfer yield. Directing such vectorial charge transfer in a photocatalytic membrane will be useful in suppressing undesired side reactions (e.g., re-oxidation of a reduced product) and facilitating product separation.
Photocatalytic membranes prepared with semiconductor nanoparticles embedded in a polymer film offer a convenient approach to direct the electron and hole flow and separate reduction and oxidation products. We have now embedded In2S3 and ZnS semiconductor nanoparticles in a Nafion membrane to induce photocatalytic reactions using visible light. In addition, we incorporated a viologen redox relay within the membrane to facilitate electron transfer to thionine (TH) dissolved in water. By inserting the photocatalytic membrane in a H-cell, we can separate the oxidation and reduction products and track the electron flow using steady-state photolysis and transient absorption spectroscopy. The enhanced charge separation in the In2S3 and ZnS heterostructure at 50:50 loading allowed us to maximize the electron-transfer yield. Directing such vectorial charge transfer in a photocatalytic membrane will be useful in suppressing undesired side reactions (e.g., re-oxidation of a reduced product) and facilitating product separation.
578. Extending Infrared Emission via Energy Transfer in a CsPbI3–Cyanine Dye Hybrid Jishnudas Chakkamalayath, Lauren E. Martin, and Prashant V. Kamat
J. Phys. Chem. Lett. 2024, 15, 2, 401-407.
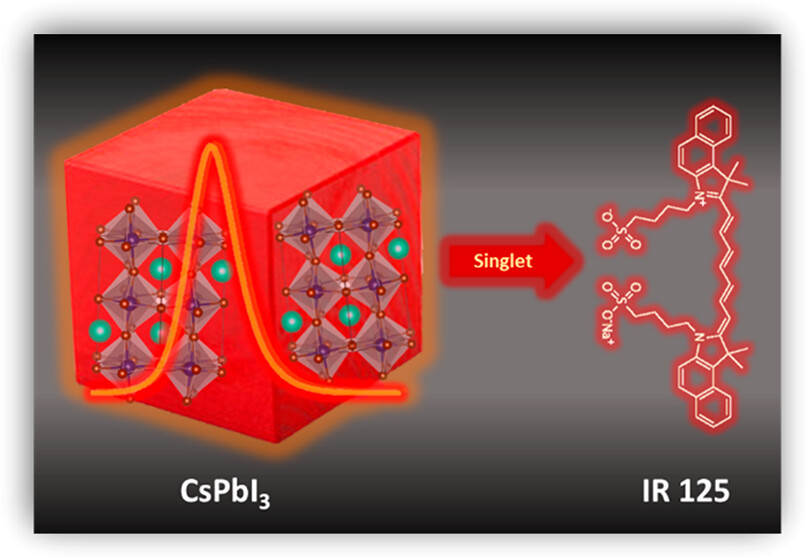 Directing energy flow in light harvesting assemblies of nanocrystal–chromophore hybrid systems requires a better understanding of factors that dictate excited-state processes. In this study, we explore excited-state interactions within the CsPbI3–cyanine dye (IR125) hybrid assembly through a comprehensive set of steady-state and time-resolved absorption and photoluminescence (PL) experiments. Our photoluminescence investigations reveal the quenching of CsPbI3 emission alongside the simultaneous enhancement of IR125 fluorescence, providing evidence for a singlet energy transfer. The evaluation of both photoluminescence (PL) quenching and PL decay measurements yield ∼94% energy transfer efficiency for the CsPbI3–IR125 hybrid assembly. Transient absorption spectroscopy further unveils that this singlet energy transfer process operates on an ultrafast time scale, occurring within 400 ps with a rate constant of energy transfer of 1.4 × 1010 s-1. Our findings highlight the potential of the CsPbI3–IR125 hybrid assembly to extend the emission of halide perovskites into the infrared region, paving the way for light energy harvesting and display applications.
Directing energy flow in light harvesting assemblies of nanocrystal–chromophore hybrid systems requires a better understanding of factors that dictate excited-state processes. In this study, we explore excited-state interactions within the CsPbI3–cyanine dye (IR125) hybrid assembly through a comprehensive set of steady-state and time-resolved absorption and photoluminescence (PL) experiments. Our photoluminescence investigations reveal the quenching of CsPbI3 emission alongside the simultaneous enhancement of IR125 fluorescence, providing evidence for a singlet energy transfer. The evaluation of both photoluminescence (PL) quenching and PL decay measurements yield ∼94% energy transfer efficiency for the CsPbI3–IR125 hybrid assembly. Transient absorption spectroscopy further unveils that this singlet energy transfer process operates on an ultrafast time scale, occurring within 400 ps with a rate constant of energy transfer of 1.4 × 1010 s-1. Our findings highlight the potential of the CsPbI3–IR125 hybrid assembly to extend the emission of halide perovskites into the infrared region, paving the way for light energy harvesting and display applications.
577. AgInS2-Embedded Photocatalytic Membrane: Insights into the Excited State and Electron Transfer Dynamics Anthony Kipkorir, Gavin Ealey, Yiseul Yu, and Prashant V. Kamat
Langmuir 2024, 40, 2, 1373-1380.
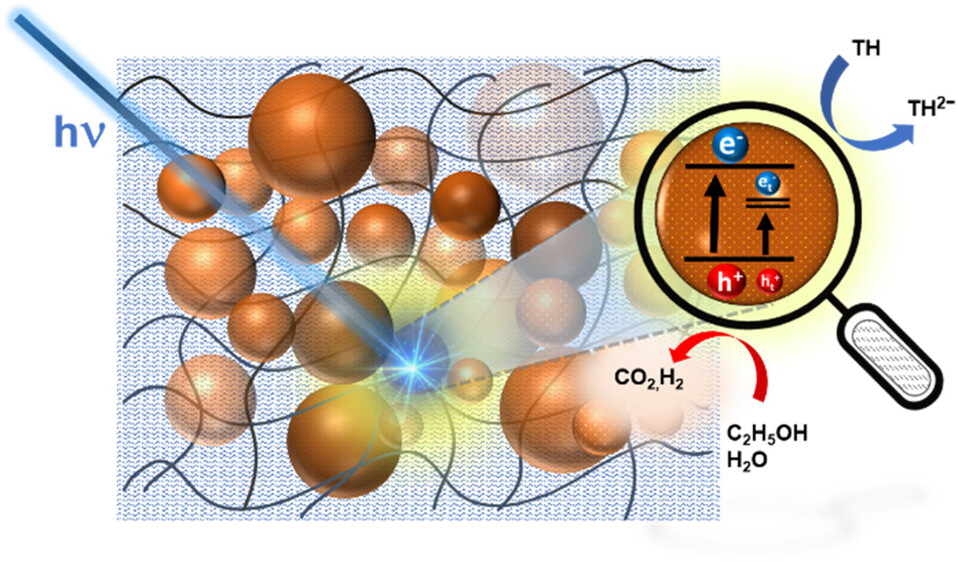 Photocatalytic reactions at semiconductor nanocrystal surfaces are useful for synthesizing value-added chemicals using sunlight. Semiconductor nanocrystals dispersed in a rigid framework, such as polymer film, can mitigate issues such as aggregation, product separation, and other challenges that are usually encountered in suspensions or slurries. Using a cation exchange technique, we successfully embedded AgInS2 nanoparticles into a Nafion matrix, termed AgInS2–Nafion. This was achieved through a galvanic exchange between In and Ag in In2S3 present within the Nafion film, enabling an adjustable Ag:In ratio for optimized photophysical properties. As in the case of colloidal suspension, the AgInS2 particles embedded in Nafion exhibit a long absorption tail, a broad emission band with a large Stokes shift, and emission lifetimes extending into the microseconds that are characteristic of donor–acceptor pairs, DAP. Remediation of surface states with the treatment of 3-mercaptopropionic acid resulted in significant enhancement in the emission yield. Charge carrier generation through bandgap excitation as well as activation of DAP states which reside within the bandgap is probed through transient absorption spectroscopy. The photocatalytic activity of AgInS2–Nafion was probed by using thionine as an electron acceptor. The electron transfer rate constant from excited AgInS2 to thionine as observed from transient absorption spectroscopy was determined to be ∼6.3 × 1010 s-1. The design of a photoactive membrane offers new ways to carry out photocatalytic processes with greater selectivity.
Photocatalytic reactions at semiconductor nanocrystal surfaces are useful for synthesizing value-added chemicals using sunlight. Semiconductor nanocrystals dispersed in a rigid framework, such as polymer film, can mitigate issues such as aggregation, product separation, and other challenges that are usually encountered in suspensions or slurries. Using a cation exchange technique, we successfully embedded AgInS2 nanoparticles into a Nafion matrix, termed AgInS2–Nafion. This was achieved through a galvanic exchange between In and Ag in In2S3 present within the Nafion film, enabling an adjustable Ag:In ratio for optimized photophysical properties. As in the case of colloidal suspension, the AgInS2 particles embedded in Nafion exhibit a long absorption tail, a broad emission band with a large Stokes shift, and emission lifetimes extending into the microseconds that are characteristic of donor–acceptor pairs, DAP. Remediation of surface states with the treatment of 3-mercaptopropionic acid resulted in significant enhancement in the emission yield. Charge carrier generation through bandgap excitation as well as activation of DAP states which reside within the bandgap is probed through transient absorption spectroscopy. The photocatalytic activity of AgInS2–Nafion was probed by using thionine as an electron acceptor. The electron transfer rate constant from excited AgInS2 to thionine as observed from transient absorption spectroscopy was determined to be ∼6.3 × 1010 s-1. The design of a photoactive membrane offers new ways to carry out photocatalytic processes with greater selectivity.
576. How Cation Migration across a 2D/3D Interface Dictates Perovskite Solar Cell Efficiency Gábor Szabó and Prashant V. Kamat
ACS Energy Lett. 2024, 9, 1, 193-200.
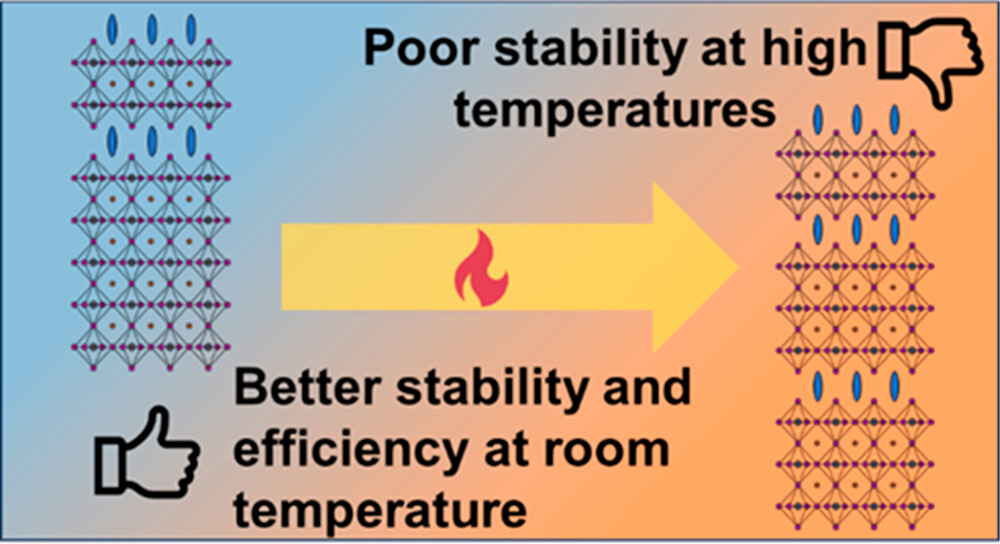 Perovskite solar cells with 2D/3D architecture are claimed to exhibit better stability compared to pristine 3D films at room temperature. However, under illumination and/or heat, cation migration causes the exchange of the bulky spacer cations (2D phase) with the smaller A-site cations (3D phase), creating a gradient heterostructure at the 2D/3D interface. We have evaluated the performance of BA2MAPb2I7/MAPbI3 (2D/3D) and MAPbI3 (3D) solar cells at different temperatures, while simultaneously probing the absorption changes of the 2D/3D perovskite layer and the photovoltaic performance of solar cell devices. The 2D/3D solar cells were more stable at room temperature but exhibited deterioration of photovoltaic performance at high temperatures. By employing in situ measurements of operating solar cells to track both the photoconversion efficiency and absorption changes at different temperatures, we show that the cation exchange at the 2D/3D interface contributes to the efficiency losses.
Perovskite solar cells with 2D/3D architecture are claimed to exhibit better stability compared to pristine 3D films at room temperature. However, under illumination and/or heat, cation migration causes the exchange of the bulky spacer cations (2D phase) with the smaller A-site cations (3D phase), creating a gradient heterostructure at the 2D/3D interface. We have evaluated the performance of BA2MAPb2I7/MAPbI3 (2D/3D) and MAPbI3 (3D) solar cells at different temperatures, while simultaneously probing the absorption changes of the 2D/3D perovskite layer and the photovoltaic performance of solar cell devices. The 2D/3D solar cells were more stable at room temperature but exhibited deterioration of photovoltaic performance at high temperatures. By employing in situ measurements of operating solar cells to track both the photoconversion efficiency and absorption changes at different temperatures, we show that the cation exchange at the 2D/3D interface contributes to the efficiency losses.
575. Cation Migration in Physically Paired 2D and 3D Lead Halide Perovskite Films Preethi S. Mathew and Prashant V. Kamat
Adv. Optical Mater. 2023, 2300957.
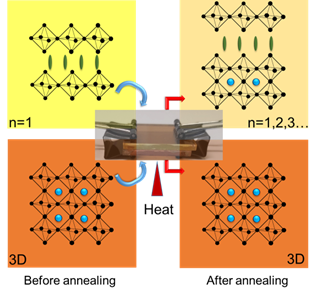 2D/3D interfaces provide long-term stability for the operation of metal halide perovskite solar cells. However, the cation migration under heat and light can disturb the interface and create a graded cation interface. This study has now probed the cation migration by physically pairing X2PbI4 (X = butylammonium BA, oleylammonium OA, or phenethylammonium PEA) 2D film and (CH3NH3, MA)PbI3 3D film at different temperatures and recording changes in the absorption and emission spectra. The migration of the methyl ammonium cation toward the 2D film slowly transforms the n = 1 layered phase into n = 2 and 3 layered phases. The 3D film, on the other hand, exhibits relatively small changes, as the inclusion of spacer cation has little effect on its phase. The apparent activation energy determined from the temperature-dependent cation migration kinetics (Ea = 29– 50 kJ mol-1) indicates alkyl ammonium cations such as butyl ammonium migrate more readily into the 3D layer than aromatic cations such as PEA. The ease of migration of spacer cations between physically paired films suggests that the varied composition at the interface should be considered while evaluating the effectiveness of the 2D/3D design strategy for improving the performance of perovskite solar cells.
2D/3D interfaces provide long-term stability for the operation of metal halide perovskite solar cells. However, the cation migration under heat and light can disturb the interface and create a graded cation interface. This study has now probed the cation migration by physically pairing X2PbI4 (X = butylammonium BA, oleylammonium OA, or phenethylammonium PEA) 2D film and (CH3NH3, MA)PbI3 3D film at different temperatures and recording changes in the absorption and emission spectra. The migration of the methyl ammonium cation toward the 2D film slowly transforms the n = 1 layered phase into n = 2 and 3 layered phases. The 3D film, on the other hand, exhibits relatively small changes, as the inclusion of spacer cation has little effect on its phase. The apparent activation energy determined from the temperature-dependent cation migration kinetics (Ea = 29– 50 kJ mol-1) indicates alkyl ammonium cations such as butyl ammonium migrate more readily into the 3D layer than aromatic cations such as PEA. The ease of migration of spacer cations between physically paired films suggests that the varied composition at the interface should be considered while evaluating the effectiveness of the 2D/3D design strategy for improving the performance of perovskite solar cells.
574. Thermodynamic Band Gap Model for Photoinduced Phase Segregation in Mixed-Halide Perovskites
Anthony Ruth, Halyna Okrepka, Prashant V. Kamat, and Masaru Kuno
J. Phys. Chem. C. 2023, 127, 37, 18547-18559
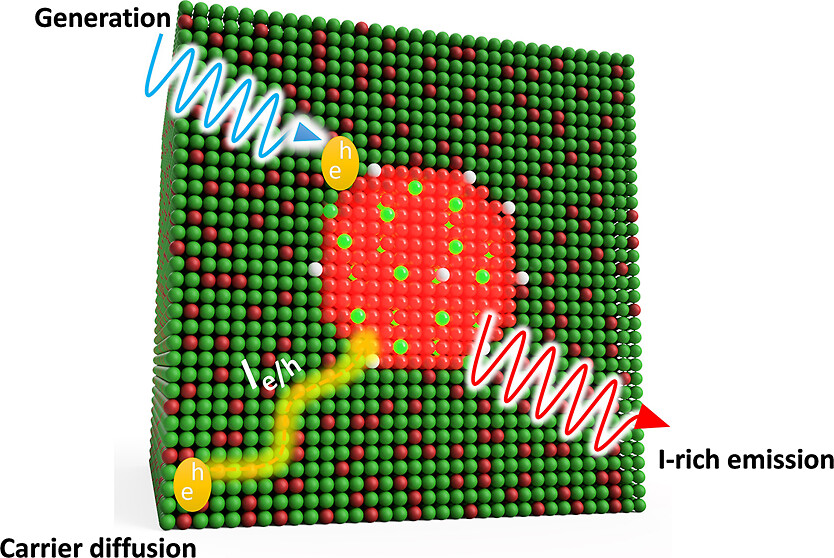 Provided is a comprehensive description of a band gap thermodynamic model, which predicts and explains key features of photosegregation in lead-based, mixed-halide perovskites. The model gives a prescription for illustrating halide migration driven by photocarrier energies. Where possible, model predictions are compared with experimental results. Free energy derivations are provided for three assumptions: (1) halide mixing in the dark, (2) a fixed number of photogenerated carriers funneling to and localizing in low band gap inclusions of the alloy, and (3) the statistical occupancy of said inclusions from a bath of thermalized photocarriers in the parent material. Model predictions include excitation intensity (Iexc)-dependent terminal halide stoichiometries (xterminal), temperature-independent xterminal, excitation intensity thresholds (Iexc,threshold) below which photosegregation is suppressed, and reduced segregation in nanocrystals as compared to thin films. The model also offers insight into kinetically manipulating photosegregation rates via control of underlying mediators and rationalizes asymmetries in forward and reverse photosegregation rate constants/activation energies. What emerges is a cohesive framework for understanding ubiquitous photosegregation in mixed-halide perovskites and a rational basis by which to manage the phenomenon.
Provided is a comprehensive description of a band gap thermodynamic model, which predicts and explains key features of photosegregation in lead-based, mixed-halide perovskites. The model gives a prescription for illustrating halide migration driven by photocarrier energies. Where possible, model predictions are compared with experimental results. Free energy derivations are provided for three assumptions: (1) halide mixing in the dark, (2) a fixed number of photogenerated carriers funneling to and localizing in low band gap inclusions of the alloy, and (3) the statistical occupancy of said inclusions from a bath of thermalized photocarriers in the parent material. Model predictions include excitation intensity (Iexc)-dependent terminal halide stoichiometries (xterminal), temperature-independent xterminal, excitation intensity thresholds (Iexc,threshold) below which photosegregation is suppressed, and reduced segregation in nanocrystals as compared to thin films. The model also offers insight into kinetically manipulating photosegregation rates via control of underlying mediators and rationalizes asymmetries in forward and reverse photosegregation rate constants/activation energies. What emerges is a cohesive framework for understanding ubiquitous photosegregation in mixed-halide perovskites and a rational basis by which to manage the phenomenon.
573. Directing Singlet Excited Energy Flow in Rubrene-Perylene Dye (DBP) Films
Jishnudas Chakkamalayath and Prashant V. Kamat
J. Phys. Chem. C. 2023, 127, 33, 16312-16318
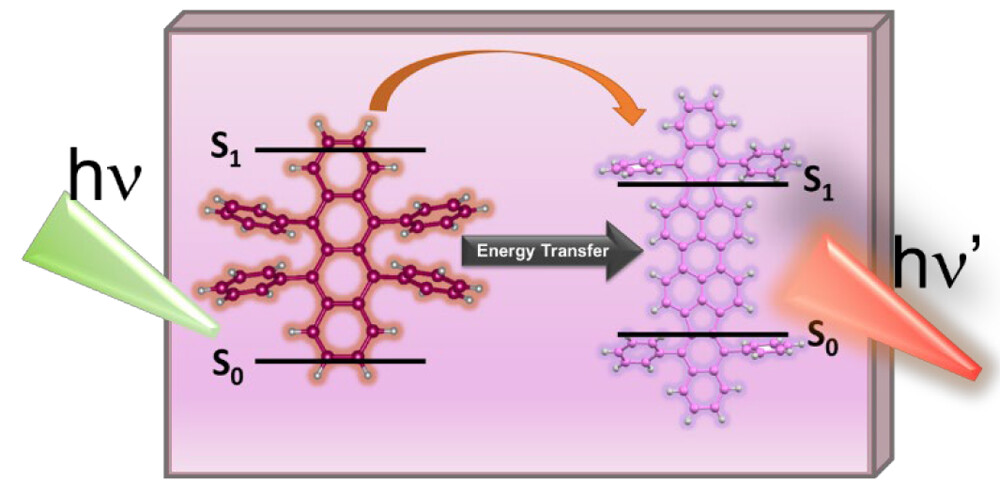 Energy transfer between a sensitizer and a triplet annihilator-emitter system is a key process that dictates the design of a light energy upconversion system. Using a combination of steady-state and time-resolved emission experiments, we have now elucidated excited-state interactions between the rubrene dye and a perylene derivative (DBP), which are commonly employed in upconversion molecular assemblies. Quenching of rubrene emission with a concomitant increase in DBP emission in rubrene-DBP films confirms singlet-energy transfer with a singlet energy transfer efficiency as high as 94%. Under low-intensity (steady-state) excitation, the singlet excited state of rubrene acts as a primary donor in the Förster resonance energy transfer process. The rate constant of energy transfer as determined from emission lifetime measurements of rubrene-DBP films was 1.4 × 109 s-1. The factors that control the singlet energy transfer between excited rubrene and DBP and its role in designing light energy harvesting assemblies are discussed.
Energy transfer between a sensitizer and a triplet annihilator-emitter system is a key process that dictates the design of a light energy upconversion system. Using a combination of steady-state and time-resolved emission experiments, we have now elucidated excited-state interactions between the rubrene dye and a perylene derivative (DBP), which are commonly employed in upconversion molecular assemblies. Quenching of rubrene emission with a concomitant increase in DBP emission in rubrene-DBP films confirms singlet-energy transfer with a singlet energy transfer efficiency as high as 94%. Under low-intensity (steady-state) excitation, the singlet excited state of rubrene acts as a primary donor in the Förster resonance energy transfer process. The rate constant of energy transfer as determined from emission lifetime measurements of rubrene-DBP films was 1.4 × 109 s-1. The factors that control the singlet energy transfer between excited rubrene and DBP and its role in designing light energy harvesting assemblies are discussed.
572. Reversible Phase Transformation of Colloidal 2D Lead Halide Perovskite Platelets under Photoirradiation
Nathaniel Hiott, Jishnudas Chakkamalayath and Prashant V. Kamat
ACS Materials Lett. 2023, 5, 10, 2614-2620
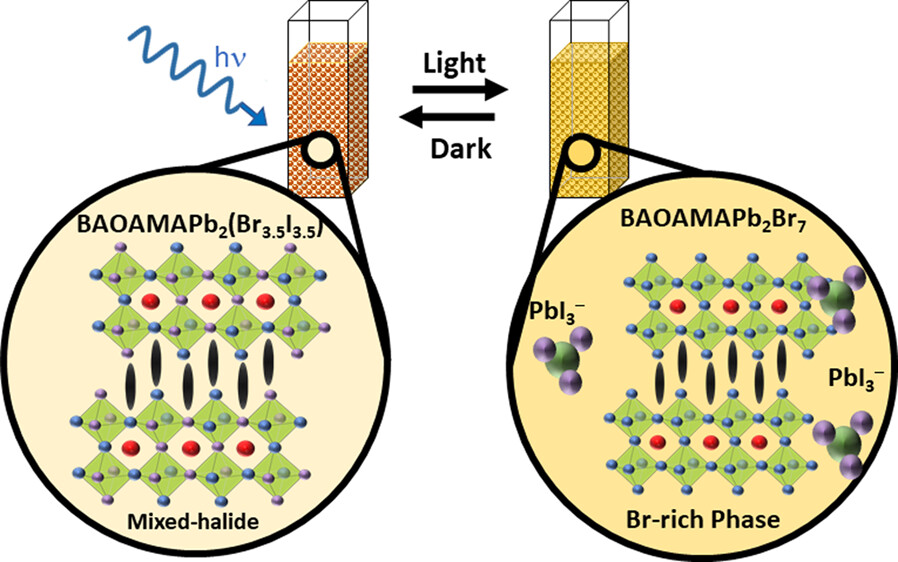 The photostability of lead halide perovskites is an important criterion for their utilization in light harvesting applications. Colloidal two-dimensional mixed halide perovskites offer a convenient approach to probe photoinduced transformations using spectroscopy tools. We employed suspensions of colloidal 2D methylammonium lead halide perovskite platelets with butylammonium and octylammonium as organic spacer cations. Upon irradiation of the mixed halide perovskite (BAOAMAPb2(Br3.5I3.5)) colloidal platelet suspension, we observe a phase transformation, as seen from the formation of bromide rich domains (BAOAMAPb2Br7) and an iodide complex (PbI3–). Unlike in the case of 3D mixed halide films, we did not observe the formation of iodide rich domains as part of the photoinduced phase segregation process. Instead, we observed the dissociation of the BAOAMAPb2I7 to form an iodide complex, PbI3–. Interestingly, this photoinduced phase transformation was reversible, as the products which were confined in the colloidal core restored the original mixed halide phase upon storage in dark. The spectroscopic measurements that characterize excited states of different colloidal methylammonium lead halide perovskites and kinetics of photoinduced transformation and their dark recovery are presented. These new phototransformations of colloidal perovskite platelets provide new insights into the processes that dictate their overall photostability.
The photostability of lead halide perovskites is an important criterion for their utilization in light harvesting applications. Colloidal two-dimensional mixed halide perovskites offer a convenient approach to probe photoinduced transformations using spectroscopy tools. We employed suspensions of colloidal 2D methylammonium lead halide perovskite platelets with butylammonium and octylammonium as organic spacer cations. Upon irradiation of the mixed halide perovskite (BAOAMAPb2(Br3.5I3.5)) colloidal platelet suspension, we observe a phase transformation, as seen from the formation of bromide rich domains (BAOAMAPb2Br7) and an iodide complex (PbI3–). Unlike in the case of 3D mixed halide films, we did not observe the formation of iodide rich domains as part of the photoinduced phase segregation process. Instead, we observed the dissociation of the BAOAMAPb2I7 to form an iodide complex, PbI3–. Interestingly, this photoinduced phase transformation was reversible, as the products which were confined in the colloidal core restored the original mixed halide phase upon storage in dark. The spectroscopic measurements that characterize excited states of different colloidal methylammonium lead halide perovskites and kinetics of photoinduced transformation and their dark recovery are presented. These new phototransformations of colloidal perovskite platelets provide new insights into the processes that dictate their overall photostability.
571. Trap or Triplet? Excited–State Interactions in 2D Perovskite Colloids with Chromophoric Cations
Jeffrey T. DuBose, Andrew Christy, Jishnudas Chakkamalayath and Prashant V. Kamat
ACS Nano 2023, 17, 19, 19052-19062
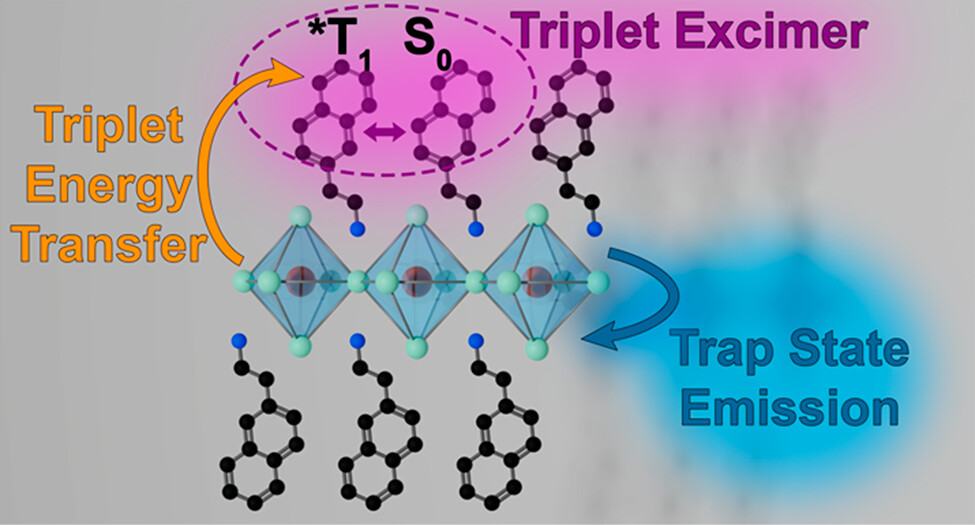 Movement of energy within light-harvesting assemblies is typically carried out with separately synthesized donor and acceptor species, which are then brought together to induce an interaction. Recently, two-dimensional (2D) lead halide perovskites have gained interest for their ability to accommodate and assemble chromophoric molecules within their lattice, creating hybrid organic–inorganic compositions. Using a combination of steady-state and time-resolved absorption and emission spectroscopy, we have now succeeded in establishing the competition between energy transfer and charge trapping in 2D halide perovskite colloids containing naphthalene-derived cations (i.e., NEA2PbX4, where NEA = naphthylethylamine). The presence of room-temperature triplet emission from the naphthalene moiety depends on the ratio of bromide to iodide in the lead halide sublattice (i.e., x in NEA2Pb(Br1–xIx)4), with only bromide-rich compositions showing sensitized emission. Photoluminescence lifetime measurements of the sensitized naphthalene reveal the formation of the naphthalene triplet excimer at room temperature. From transient absorption measurements, we find the rate constant of triplet energy transfer (kEnT) to be on the order of ∼109 s–1. At low temperatures (77 K) a new broad emission feature arising from trap states is observed in all samples ranging from pure bromide to pure iodide composition. These results reveal the interplay between sensitized triplet energy transfer and charge trapping in 2D lead halide perovskites, highlighting the need to carefully parse contributions from competing de-excitation pathways for optoelectronic applications.
Movement of energy within light-harvesting assemblies is typically carried out with separately synthesized donor and acceptor species, which are then brought together to induce an interaction. Recently, two-dimensional (2D) lead halide perovskites have gained interest for their ability to accommodate and assemble chromophoric molecules within their lattice, creating hybrid organic–inorganic compositions. Using a combination of steady-state and time-resolved absorption and emission spectroscopy, we have now succeeded in establishing the competition between energy transfer and charge trapping in 2D halide perovskite colloids containing naphthalene-derived cations (i.e., NEA2PbX4, where NEA = naphthylethylamine). The presence of room-temperature triplet emission from the naphthalene moiety depends on the ratio of bromide to iodide in the lead halide sublattice (i.e., x in NEA2Pb(Br1–xIx)4), with only bromide-rich compositions showing sensitized emission. Photoluminescence lifetime measurements of the sensitized naphthalene reveal the formation of the naphthalene triplet excimer at room temperature. From transient absorption measurements, we find the rate constant of triplet energy transfer (kEnT) to be on the order of ∼109 s–1. At low temperatures (77 K) a new broad emission feature arising from trap states is observed in all samples ranging from pure bromide to pure iodide composition. These results reveal the interplay between sensitized triplet energy transfer and charge trapping in 2D lead halide perovskites, highlighting the need to carefully parse contributions from competing de-excitation pathways for optoelectronic applications.
570. Photoinduced electron transfer across the polymer-capped CsPbBr3 interface in a polar medium
Anthony Kipkorir, Xiuyu Jin, Haifeng Gao and Prashant V. Kamat
J. Chem. Phys. 2023, 158, 14, 144702
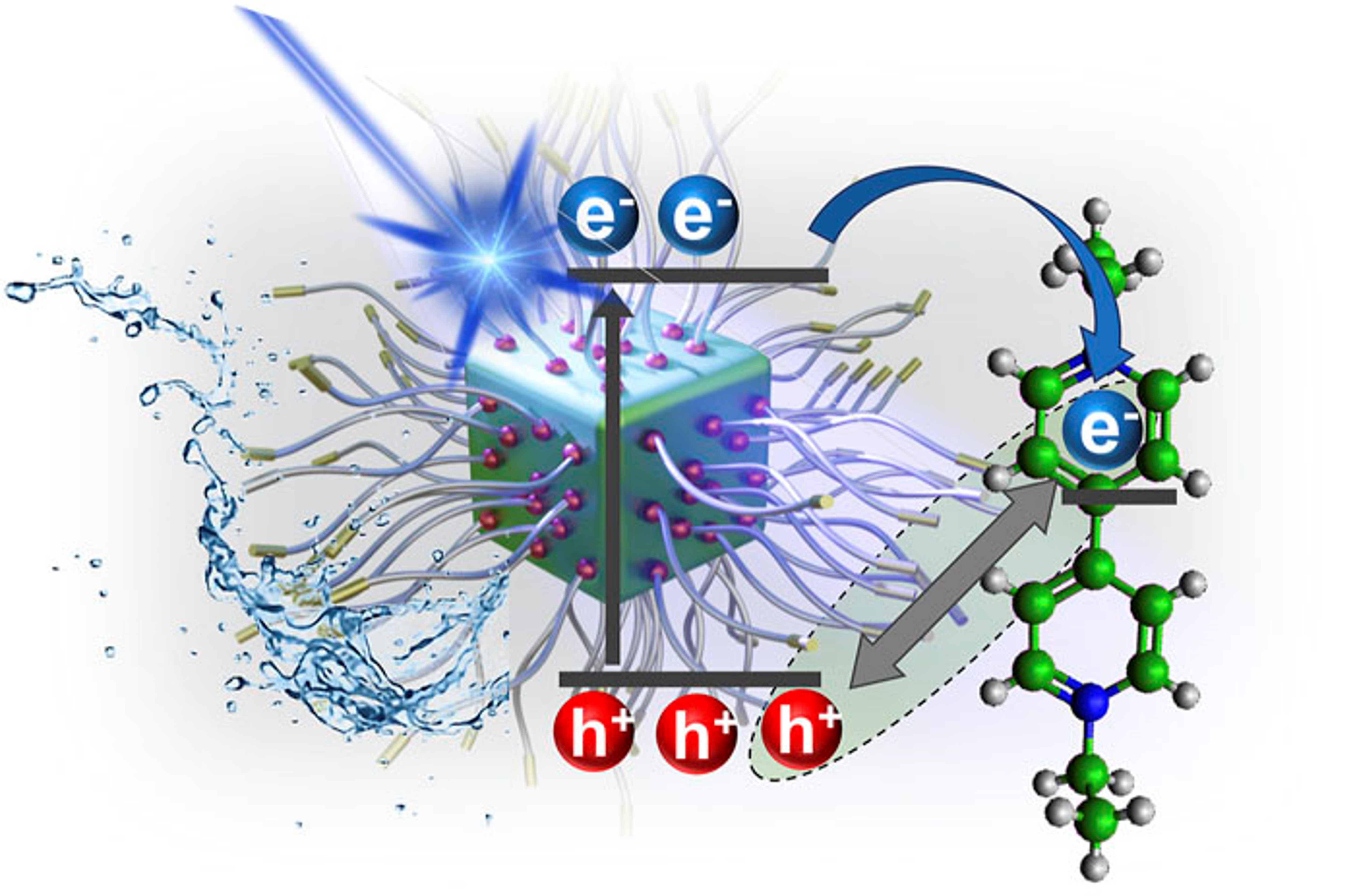 In-situ polymer capping of cesium lead bromide (CsPbBr3) nanocrystals with polymethyl acrylate is an effective approach to improve the colloidal stability in the polar medium and thus extends their use in photocatalysis. The photoinduced electron transfer properties of polymethyl acrylate (PMA)-capped CsPbBr3 nanocrystals have been probed using surface-bound viologen molecules with different alkyl chains as electron acceptors. The apparent association constant (Kapp) obtained for the binding of viologen molecules with PMA-capped CsPbBr3 was 2.3 × 107 M-1, which is an order of magnitude greater than that obtained with oleic acid/oleylamine-capped CsPbBr3. Although the length of the alkyl chain of the viologen molecule did not show any impact on the electron transfer rate constant, it influenced the charge separation efficiency and net electron transfer quantum yield. Viologen moieties with a shorter alkyl chain length exhibited a charge separation efficiency of 72% compared with 50% for the longer chain alkyl chain length viologens. Implications of polymer-capped CsPbBr3 perovskite nanocrystals for carrying out photocatalytic reduction in the polar medium are discussed.
In-situ polymer capping of cesium lead bromide (CsPbBr3) nanocrystals with polymethyl acrylate is an effective approach to improve the colloidal stability in the polar medium and thus extends their use in photocatalysis. The photoinduced electron transfer properties of polymethyl acrylate (PMA)-capped CsPbBr3 nanocrystals have been probed using surface-bound viologen molecules with different alkyl chains as electron acceptors. The apparent association constant (Kapp) obtained for the binding of viologen molecules with PMA-capped CsPbBr3 was 2.3 × 107 M-1, which is an order of magnitude greater than that obtained with oleic acid/oleylamine-capped CsPbBr3. Although the length of the alkyl chain of the viologen molecule did not show any impact on the electron transfer rate constant, it influenced the charge separation efficiency and net electron transfer quantum yield. Viologen moieties with a shorter alkyl chain length exhibited a charge separation efficiency of 72% compared with 50% for the longer chain alkyl chain length viologens. Implications of polymer-capped CsPbBr3 perovskite nanocrystals for carrying out photocatalytic reduction in the polar medium are discussed.
569. How Pendant Groups Dictate Energy and Electron Transfer in Perovskite–Rhodamine Light Harvesting Assemblies
Jeffrey T. DuBose and Prashant V. Kamat
J. Am. Chem. Soc. 2023, 145, 8, 4601-4612
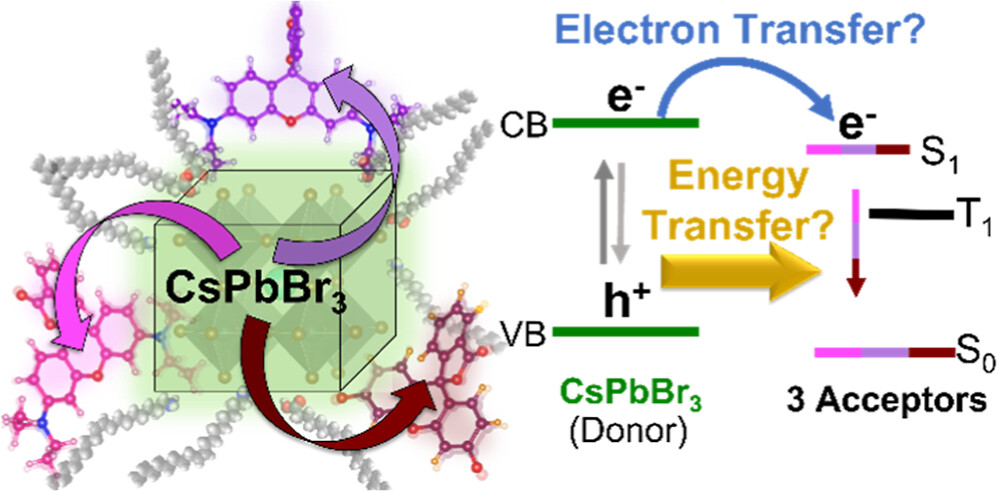 Energy and electron transfer processes allow for efficient manipulation of excited states within light harvesting assemblies for photocatalytic and optoelectronic applications. We have now successfully probed the influence of acceptor pendant group functionalization on the energy and electron transfer between CsPbBr3 perovskite nanocrystals and three rhodamine-based acceptor molecules. The three acceptors─rhodamine B (RhB), rhodamine isothiocyanate (RhB-NCS), and rose Bengal (RoseB)─contain an increasing degree of pendant group functionalization that affects their native excited state properties. When interacting with CsPbBr3 as an energy donor, photoluminescence excitation spectroscopy reveals that singlet energy transfer occurs with all three acceptors. However, the acceptor functionalization directly influences several key parameters that dictate the excited state interactions. For example, RoseB binds to the nanocrystal surface with an apparent association constant (Kapp = 9.4 × 106 M-1) 200 times greater than RhB (Kapp = 0.05 × 106 M-1), thus influencing the rate of energy transfer. Femtosecond transient absorption reveals the observed rate constant of singlet energy transfer (kEnT) is an order-of-magnitude greater for RoseB (kEnT = 1 × 1011 s-1) than for RhB and RhB-NCS. In addition to energy transfer, each acceptor had a subpopulation of molecules (∼30%) that underwent electron transfer as a competing pathway. Thus, the structural influence of acceptor moieties must be considered for both excited state energy and electron transfer in nanocrystal-molecular hybrids. The competition between electron and energy transfer further highlights the complexity of excited state interactions in nanocrystal-molecular complexes and the need for careful spectroscopic analysis to elucidate competitive pathways.
Energy and electron transfer processes allow for efficient manipulation of excited states within light harvesting assemblies for photocatalytic and optoelectronic applications. We have now successfully probed the influence of acceptor pendant group functionalization on the energy and electron transfer between CsPbBr3 perovskite nanocrystals and three rhodamine-based acceptor molecules. The three acceptors─rhodamine B (RhB), rhodamine isothiocyanate (RhB-NCS), and rose Bengal (RoseB)─contain an increasing degree of pendant group functionalization that affects their native excited state properties. When interacting with CsPbBr3 as an energy donor, photoluminescence excitation spectroscopy reveals that singlet energy transfer occurs with all three acceptors. However, the acceptor functionalization directly influences several key parameters that dictate the excited state interactions. For example, RoseB binds to the nanocrystal surface with an apparent association constant (Kapp = 9.4 × 106 M-1) 200 times greater than RhB (Kapp = 0.05 × 106 M-1), thus influencing the rate of energy transfer. Femtosecond transient absorption reveals the observed rate constant of singlet energy transfer (kEnT) is an order-of-magnitude greater for RoseB (kEnT = 1 × 1011 s-1) than for RhB and RhB-NCS. In addition to energy transfer, each acceptor had a subpopulation of molecules (∼30%) that underwent electron transfer as a competing pathway. Thus, the structural influence of acceptor moieties must be considered for both excited state energy and electron transfer in nanocrystal-molecular hybrids. The competition between electron and energy transfer further highlights the complexity of excited state interactions in nanocrystal-molecular complexes and the need for careful spectroscopic analysis to elucidate competitive pathways.
568. How Stable Is the 2D/3D Interface of Metal Halide Perovskite under Light and Heat?
Jishnudas Chakkamalayath, Nathaniel Hiott, and Prashant V. Kamat
ACS Energy Lett. 2023, 8, 1, 169-171
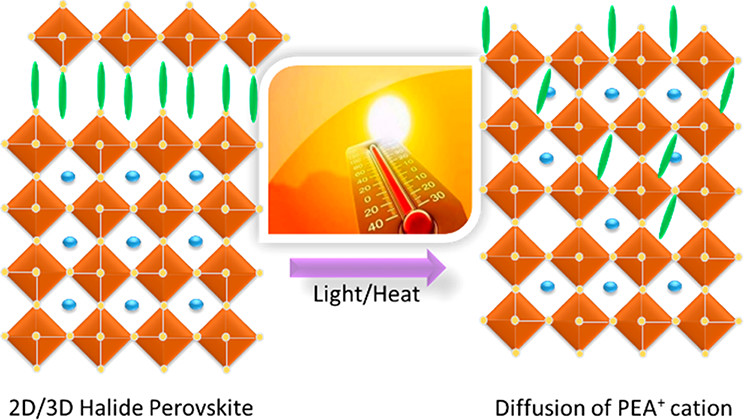 A (PEA)2PbI4 (2D) layer deposited on a MAPbI3 (3D) film undergoes a transformation upon photoirradiation and thermal treatment. The decrease in absorption and emission of the 2D layer during light and heat exposure, which shows migration of the PEA+ cation within the 3D film, points out the dynamic nature of the 2D/3D interface and intermixing of the two phases.
A (PEA)2PbI4 (2D) layer deposited on a MAPbI3 (3D) film undergoes a transformation upon photoirradiation and thermal treatment. The decrease in absorption and emission of the 2D layer during light and heat exposure, which shows migration of the PEA+ cation within the 3D film, points out the dynamic nature of the 2D/3D interface and intermixing of the two phases.
567. Excited State and Transient Chemistry of a Perylene Derivative (DBP). An Untold Story
Jishnudas Chakkamalayath, Gábor Szabó, Jeffrey T. DuBose, and Prashant V. Kamat
J. Phys. Chem. A 2023, 127, 1, 99-106
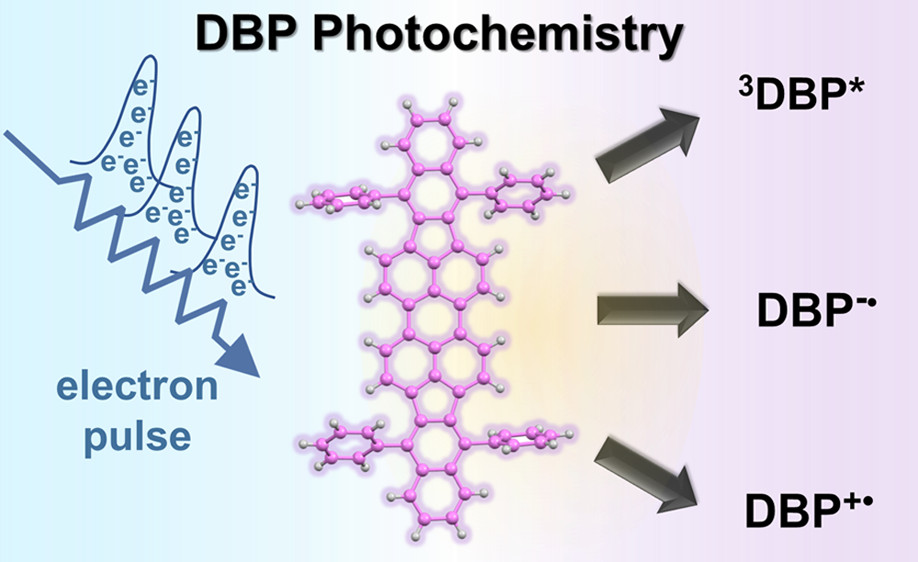 Transient chemistry of sensitizing dyes is important to obtain insights into the photochemical conversion processes of light harvesting assemblies. We have now employed transient absorption spectroscopy (pulsed laser and pulse radiolysis) to characterize the excited state and radical intermediates of a perylene derivative, (5,10,15,20-Tetraphenylbisbenz[5,6]indeno[1,2,3-cd:1′,2′,3′-lm]perylene (DBP). The distinguishable transient absorption features for the singlet and triplet excited states and radical anion and radical cation provide spectral fingerprints to identify the reaction intermediates in photochemical energy and electron transfer processes of composite systems involving DBP. For example, identifying these transients in the energy transfer processes of the rubrene–DBP system would aid in establishing their role as annihilator-emitter for triplet–triplet annihilation up-conversion (TTA-UC). The transient characterization thus serves as an important mechanistic fingerprint for elucidating mechanistic details of systems employing DBP in optoelectronic applications.
Transient chemistry of sensitizing dyes is important to obtain insights into the photochemical conversion processes of light harvesting assemblies. We have now employed transient absorption spectroscopy (pulsed laser and pulse radiolysis) to characterize the excited state and radical intermediates of a perylene derivative, (5,10,15,20-Tetraphenylbisbenz[5,6]indeno[1,2,3-cd:1′,2′,3′-lm]perylene (DBP). The distinguishable transient absorption features for the singlet and triplet excited states and radical anion and radical cation provide spectral fingerprints to identify the reaction intermediates in photochemical energy and electron transfer processes of composite systems involving DBP. For example, identifying these transients in the energy transfer processes of the rubrene–DBP system would aid in establishing their role as annihilator-emitter for triplet–triplet annihilation up-conversion (TTA-UC). The transient characterization thus serves as an important mechanistic fingerprint for elucidating mechanistic details of systems employing DBP in optoelectronic applications.
566. Enhanced Charge Carrier Separation in WO3/BiVO4 Photoanodes Achieved via Light Absorption in the BiVO4 Layer
Ivan Grigioni, Annalisa Polo, Maria Vittoria Dozzi, Kevin G. Stamplecoskie, Danilo H. Jara,
Prashant V. Kamat, and Elena Selli
ACS Appl. Energy Mater. 2022, 5, 11, 13142-13148
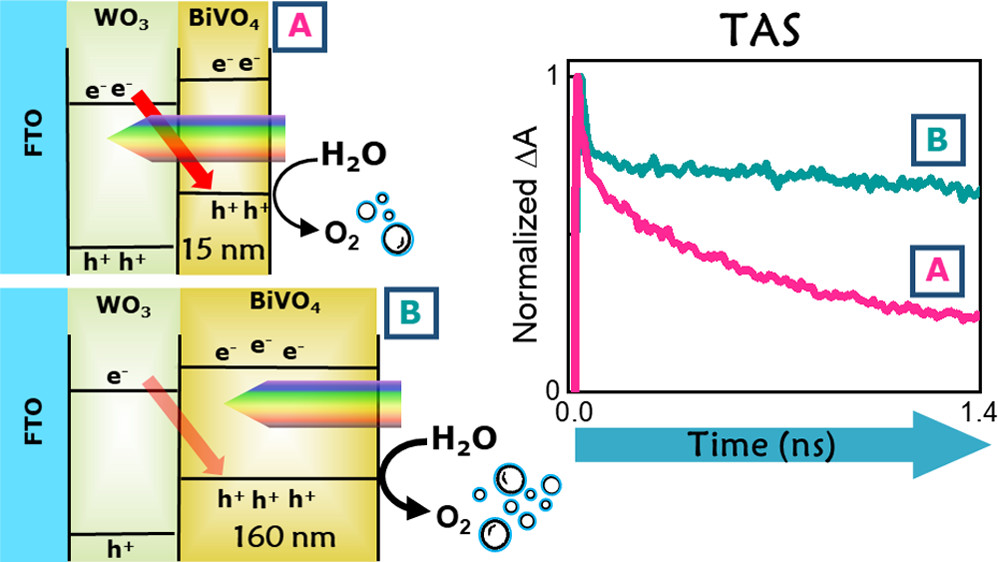 Photoelectrochemical (PEC) water splitting converts solar light and water into oxygen and energy-rich hydrogen. WO3/BiVO4 heterojunction photoanodes perform much better than the separate oxide components, though internal charge recombination undermines their PEC performance when both oxides absorb light. Here we exploit the BiVO4 layer to sensitize WO4 to visible light and shield it from direct photoexcitation to overcome this efficiency loss. PEC experiments and ultrafast transient absorption spectroscopy performed by frontside (through BiVO4) or backside (through WO3) irradiating photoanodes with different BiVO4 layer thickness demonstrate that irradiation through BiVO4 is beneficial for charge separation. Optimized electrodes irradiated through BiVO4 show 40% higher photocurrent density compared to backside irradiation.
Photoelectrochemical (PEC) water splitting converts solar light and water into oxygen and energy-rich hydrogen. WO3/BiVO4 heterojunction photoanodes perform much better than the separate oxide components, though internal charge recombination undermines their PEC performance when both oxides absorb light. Here we exploit the BiVO4 layer to sensitize WO4 to visible light and shield it from direct photoexcitation to overcome this efficiency loss. PEC experiments and ultrafast transient absorption spectroscopy performed by frontside (through BiVO4) or backside (through WO3) irradiating photoanodes with different BiVO4 layer thickness demonstrate that irradiation through BiVO4 is beneficial for charge separation. Optimized electrodes irradiated through BiVO4 show 40% higher photocurrent density compared to backside irradiation.
565. Phase Segregation and Sequential Expulsion of Iodide and Bromide in Photoirradiated Ruddlesden–Popper 2D Perovskite Films
Preethi Susan Mathew, Gábor Szabó, Masaru Kuno, and Prashant V. Kamat
ACS Energy Lett. 2022, 7, 11, 3982–3988
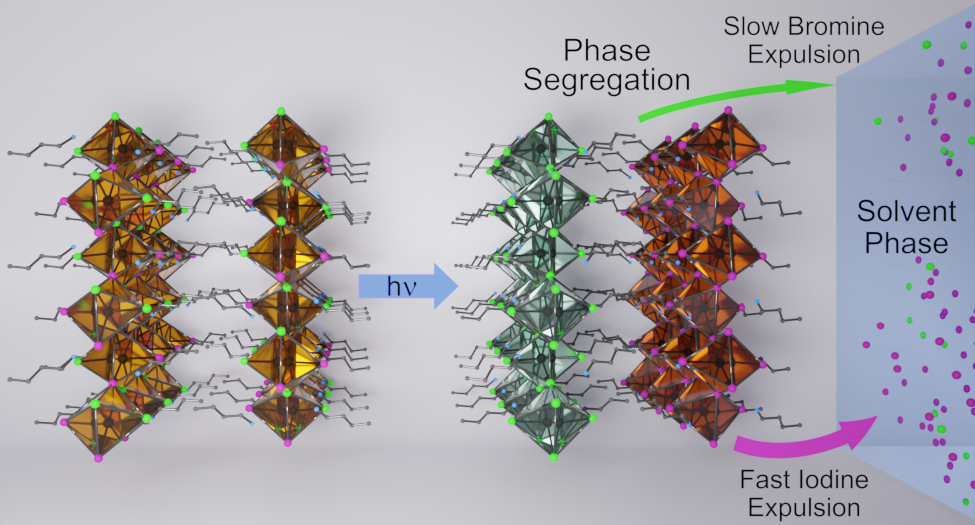 Two-dimensional (2D) Ruddlesden–Popper mixed-halide perovskite films, BA2PbBr2I2, undergo phase segregation when excited with visible light to generate bromide- and iodide-rich regions, as marked by absorption and emission changes. Upon stopping illumination, the process reverses, allowing original film compositions to be restored. However, if films are in contact with dichloromethane, light irradiation causes the sequential expulsion of iodide and bromide and introduces irreversible changes to the 2D films. The sequential disappearance of I- and Br- from pristine films (BA2PbBr4 and BA2PbI4) under photoirradiation, as observed from variances in expulsion rates, reflects differences in halide ion mobilities in these films. The photoinstability of 2D films raises questions about their use in stabilizing bulk, three-dimensional (3D) perovskite solar cells through 3D/2D interfaces.
Two-dimensional (2D) Ruddlesden–Popper mixed-halide perovskite films, BA2PbBr2I2, undergo phase segregation when excited with visible light to generate bromide- and iodide-rich regions, as marked by absorption and emission changes. Upon stopping illumination, the process reverses, allowing original film compositions to be restored. However, if films are in contact with dichloromethane, light irradiation causes the sequential expulsion of iodide and bromide and introduces irreversible changes to the 2D films. The sequential disappearance of I- and Br- from pristine films (BA2PbBr4 and BA2PbI4) under photoirradiation, as observed from variances in expulsion rates, reflects differences in halide ion mobilities in these films. The photoinstability of 2D films raises questions about their use in stabilizing bulk, three-dimensional (3D) perovskite solar cells through 3D/2D interfaces.

 * Source: Web of Science - 05/02/2024
* Source: Web of Science - 05/02/2024

 Extending the lifetime of photogenerated electrons in semiconductor systems is an important criterion for the conversion of light into storable energy. We have now succeeded in storing electrons in a photoirradiated colloidal molybdenum disulfide (MoS2) suspension, showcasing its unique reversible photoresponsive behavior. The dampened A and B excitonic peaks indicate the accumulation of photogenerated electrons and the minimization of interactions between MoS2 interlayers. The stored electrons were quantitatively extracted by titrating with a ferrocenium ion in the dark, giving ca. 0.2 electrons per MoS2 formula unit. The emergence of the photoinduced A1g* Raman mode and the decrease in zeta potential after irradiation suggest intercalation of counterions to maintain overall charge balance upon electron storage. These results provide insights into the mechanism of photogenerated electron storage in 2D materials and pave the way for the potential application of colloidal 2D materials in electron storage.
Extending the lifetime of photogenerated electrons in semiconductor systems is an important criterion for the conversion of light into storable energy. We have now succeeded in storing electrons in a photoirradiated colloidal molybdenum disulfide (MoS2) suspension, showcasing its unique reversible photoresponsive behavior. The dampened A and B excitonic peaks indicate the accumulation of photogenerated electrons and the minimization of interactions between MoS2 interlayers. The stored electrons were quantitatively extracted by titrating with a ferrocenium ion in the dark, giving ca. 0.2 electrons per MoS2 formula unit. The emergence of the photoinduced A1g* Raman mode and the decrease in zeta potential after irradiation suggest intercalation of counterions to maintain overall charge balance upon electron storage. These results provide insights into the mechanism of photogenerated electron storage in 2D materials and pave the way for the potential application of colloidal 2D materials in electron storage.
 Ternary I–III–VI2 semiconductors, such as CuInS2 and AgInS2 (compliant with RoHS, restriction of hazardous substances), are useful as light-harvesting materials. However, the presence of sub-bandgap states (donor–acceptor pair or DAP) introduces complexity during their activation through photoexcitation. When photoirradiated, the photogenerated charge carriers in AgInS2 quantum dots undergo rapid relaxation to populate intrinsic DAP states while competing with charge carrier recombination. Interestingly, these defect-related DAP states can be activated through sub-bandgap excitation and, thus, extend the absorption range to the near-infrared region. We have now employed time-resolved absorption and emission techniques to glean mechanistic insights into the photophysical properties of intragap states of AgInS2 quantum dots (QDs) and their participation in interfacial electron transfer. When the AgInS2 QDs are excited with above bandgap excitation (400 nm), we observe a prompt formation (< 1 ps) of the bleach at wavelengths closer to the bandgap, indicating the formation of a charge-separated pair. This transient bleach shifts to lower energies with time (∼5 ps), indicating population of sub-bandgap states via relaxation of electrons and holes from the conduction and valence bands, respectively. These sub-bandgap states which can also be populated via direct excitation using low energy (λ < Eg) excitation exhibit prompt bleach (< 1 ps) in contrast to bandgap excitation. The excited DAP states are long-lived (∼1 μs) and can participate in the electron transfer process. We have elucidated the electron transfer dynamics from these midgap states of AgInS2 by employing ethyl viologen (EV2+) as a probe molecule. The role of surface-anchored viologen as an electron shuttle was further exploited by using free-floating benzoquinone (BQ) as a secondary electron acceptor. The sub-bandgap response of AgInS2 to promote electron transfer paves the way to extend the photoresponse of ternary I–III–VI2 semiconductor-based photocatalytic systems.
Ternary I–III–VI2 semiconductors, such as CuInS2 and AgInS2 (compliant with RoHS, restriction of hazardous substances), are useful as light-harvesting materials. However, the presence of sub-bandgap states (donor–acceptor pair or DAP) introduces complexity during their activation through photoexcitation. When photoirradiated, the photogenerated charge carriers in AgInS2 quantum dots undergo rapid relaxation to populate intrinsic DAP states while competing with charge carrier recombination. Interestingly, these defect-related DAP states can be activated through sub-bandgap excitation and, thus, extend the absorption range to the near-infrared region. We have now employed time-resolved absorption and emission techniques to glean mechanistic insights into the photophysical properties of intragap states of AgInS2 quantum dots (QDs) and their participation in interfacial electron transfer. When the AgInS2 QDs are excited with above bandgap excitation (400 nm), we observe a prompt formation (< 1 ps) of the bleach at wavelengths closer to the bandgap, indicating the formation of a charge-separated pair. This transient bleach shifts to lower energies with time (∼5 ps), indicating population of sub-bandgap states via relaxation of electrons and holes from the conduction and valence bands, respectively. These sub-bandgap states which can also be populated via direct excitation using low energy (λ < Eg) excitation exhibit prompt bleach (< 1 ps) in contrast to bandgap excitation. The excited DAP states are long-lived (∼1 μs) and can participate in the electron transfer process. We have elucidated the electron transfer dynamics from these midgap states of AgInS2 by employing ethyl viologen (EV2+) as a probe molecule. The role of surface-anchored viologen as an electron shuttle was further exploited by using free-floating benzoquinone (BQ) as a secondary electron acceptor. The sub-bandgap response of AgInS2 to promote electron transfer paves the way to extend the photoresponse of ternary I–III–VI2 semiconductor-based photocatalytic systems.
 A photocatalytically active membrane, designed by embedding AgInS2 semiconductor nanoparticles and a Pt cocatalyst, facilitates a “vectorial” flow of photogenerated electrons and holes in opposite directions. The fabricated Pt–AgInS2–Nafion membrane, when inserted in an H-cell containing 50% ethanol (AgInS2 side) and water (pH = 4, Pt side), produced H2 under visible light irradiation. Photogenerated electrons reduced H+ at the Pt surface to produce H2, while oxidation of ethanol with holes at AgInS2 also produced H2. Back electron transfer at the Pt surface and surface defects within AgInS2 were responsible for the lower H2 yield in the reduction compartment. Remediation of the AgInS2 film with mercaptopropionic acid increased the yield 5–10 times by overcoming the loss of charge carriers at the defect sites. The feasibility of carrying out selective reduction and oxidation processes by directing the flow of charge carriers highlights the usefulness of photocatalytic membranes in solar fuel generation.
A photocatalytically active membrane, designed by embedding AgInS2 semiconductor nanoparticles and a Pt cocatalyst, facilitates a “vectorial” flow of photogenerated electrons and holes in opposite directions. The fabricated Pt–AgInS2–Nafion membrane, when inserted in an H-cell containing 50% ethanol (AgInS2 side) and water (pH = 4, Pt side), produced H2 under visible light irradiation. Photogenerated electrons reduced H+ at the Pt surface to produce H2, while oxidation of ethanol with holes at AgInS2 also produced H2. Back electron transfer at the Pt surface and surface defects within AgInS2 were responsible for the lower H2 yield in the reduction compartment. Remediation of the AgInS2 film with mercaptopropionic acid increased the yield 5–10 times by overcoming the loss of charge carriers at the defect sites. The feasibility of carrying out selective reduction and oxidation processes by directing the flow of charge carriers highlights the usefulness of photocatalytic membranes in solar fuel generation.
 The capability of halide perovskite nanocrystals to sensitize both singlet and triplet excited states of a chromophore highlights their potential as photosensitizers in down conversion (singlet energy transfer) or upconversion (triplet energy transfer followed by triplet–triplet annihilation) applications. In semiconductor-multiple chromophore assemblies, however, various possibilities arise to modulate the energy transfer process and hence the final emission output. By employing CsPbBr3 nanocrystals (Eg = 2.47 eV) as a primary sensitizer, we have now probed the singlet energy transfer to two dyes, viz., rubrene (Es = 2.23 eV) and DBP, a perylene derivative (Es = 2.07 eV). By first characterizing the excited-state interactions between CsPbBr3 and individual dye pairs, we establish the favorable pathway for singlet energy transfer in a donor–acceptor1–acceptor2 assembly. The singlet energy transfer efficiencies for CsPbBr3-rubrene, rubrene-DBP, and CsPbBr3-DBP systems were quantified as 89%, 94%, and 28%, respectively. When all three components (CsPbBr3-rubrene-DBP) are present in the film, we observe a cascading energy transfer to yield a high population of singlet excited-states in DBP. The 1DBP* yield increased with rubrene concentration, thus confirming its mediating role in the energy cascade. Thus, a proper choice of mediator can promote singlet energy transfer when the spectral overlap between a donor and acceptor is poor. Elucidation of the excited-state interactions in CsPbBr3-rubrene DBP films offers new insights into the sensitization of multiple chromophore assemblies and ways to modulate the singlet energy flow.
The capability of halide perovskite nanocrystals to sensitize both singlet and triplet excited states of a chromophore highlights their potential as photosensitizers in down conversion (singlet energy transfer) or upconversion (triplet energy transfer followed by triplet–triplet annihilation) applications. In semiconductor-multiple chromophore assemblies, however, various possibilities arise to modulate the energy transfer process and hence the final emission output. By employing CsPbBr3 nanocrystals (Eg = 2.47 eV) as a primary sensitizer, we have now probed the singlet energy transfer to two dyes, viz., rubrene (Es = 2.23 eV) and DBP, a perylene derivative (Es = 2.07 eV). By first characterizing the excited-state interactions between CsPbBr3 and individual dye pairs, we establish the favorable pathway for singlet energy transfer in a donor–acceptor1–acceptor2 assembly. The singlet energy transfer efficiencies for CsPbBr3-rubrene, rubrene-DBP, and CsPbBr3-DBP systems were quantified as 89%, 94%, and 28%, respectively. When all three components (CsPbBr3-rubrene-DBP) are present in the film, we observe a cascading energy transfer to yield a high population of singlet excited-states in DBP. The 1DBP* yield increased with rubrene concentration, thus confirming its mediating role in the energy cascade. Thus, a proper choice of mediator can promote singlet energy transfer when the spectral overlap between a donor and acceptor is poor. Elucidation of the excited-state interactions in CsPbBr3-rubrene DBP films offers new insights into the sensitization of multiple chromophore assemblies and ways to modulate the singlet energy flow.
 Lower dimensional or 2D halide perovskites, with their versatile structural and functional properties, are known to improve the performance and room temperature stability of perovskite solar cells. One would expect 2D perovskites to be more resistant to ion migration compared to their 3D counterparts because of the presence of bulky organic cations. However, recent findings show that ion migration indeed is prevalent in 2D halide perovskites similar to 3D perovskites. Halide ion migration in 2D perovskites manifests itself in halide ion segregation under photoirradiation as well as in halide exchange between physically paired films of 2D perovskites with different halide ions. Besides halide ion migration, cation migration of spacer cations and A-site cations is also seen when 2D/3D perovskite films are subjected to light and thermal stress. It is important to recognize the importance of ion migration while incorporating 2D perovskites in solar cells and other optoelectronic devices, as it can be detrimental for achieving streamlined performance and long-term stability. This Perspective discusses recent reports on ion migration in 2D and in 2D/3D halide perovskite films under the operational conditions (at elevated temperature and given built-in bias) and presents a few mitigating strategies.
Lower dimensional or 2D halide perovskites, with their versatile structural and functional properties, are known to improve the performance and room temperature stability of perovskite solar cells. One would expect 2D perovskites to be more resistant to ion migration compared to their 3D counterparts because of the presence of bulky organic cations. However, recent findings show that ion migration indeed is prevalent in 2D halide perovskites similar to 3D perovskites. Halide ion migration in 2D perovskites manifests itself in halide ion segregation under photoirradiation as well as in halide exchange between physically paired films of 2D perovskites with different halide ions. Besides halide ion migration, cation migration of spacer cations and A-site cations is also seen when 2D/3D perovskite films are subjected to light and thermal stress. It is important to recognize the importance of ion migration while incorporating 2D perovskites in solar cells and other optoelectronic devices, as it can be detrimental for achieving streamlined performance and long-term stability. This Perspective discusses recent reports on ion migration in 2D and in 2D/3D halide perovskite films under the operational conditions (at elevated temperature and given built-in bias) and presents a few mitigating strategies.
 Lead halide perovskite nanocrystals, which offer rich photochemistry, have the potential to capture photons over a wide range of the visible and infrared spectrum for photocatalytic, optoelectronic, and photon conversion applications. Energy transfer from the perovskite nanocrystal to an acceptor dye in the form of a triplet or singlet state offers additional opportunities to tune the properties of the semiconductor–dye hybrid and extend excited-state lifetimes. We have now successfully established the key factors that dictate triplet energy transfer between excited CsPbI3 and surface-bound rhodamine dyes using absorption and emission spectroscopies. The pendant groups on the acceptor dyes influence surface binding to the nanocrystals, which in turn dictate the energy transfer kinetics, as well as the efficiency of energy transfer. Of the three rhodamine dyes investigated (rhodamine B, rhodamine B isothiocyanate, and rose Bengal), the CsPbI3–rose Bengal hybrid with the strongest binding showed the highest triplet energy transfer efficiency (96%) with a rate constant of 1 × 109 s-1. This triplet energy transfer rate constant is nearly 2 orders of magnitude slower than the singlet energy transfer observed for the pure-bromide CsPbBr3–rose Bengal hybrid (1.1 × 1011 s-1). Intriguingly, although the single-halide CsPbBr3 and CsPbI3 nanocrystals selectively populate singlet and triplet excited states of rose Bengal, respectively, the mixed halide perovskites were able to generate a mixture of both singlet and triplet excited states. By tuning the bromide/iodide ratio and thus bandgap energy in CsPb(Br1-xIx)3 compositions, the percentage of singlets vs triplets delivered to the acceptor dye was systematically tuned from 0 to 100%. The excited-state properties of halide perovskite-molecular hybrids discussed here provide new ways to modulate singlet and triplet energy transfer in semiconductor–molecular dye hybrids through acceptor functionalization and donor bandgap engineering.
Lead halide perovskite nanocrystals, which offer rich photochemistry, have the potential to capture photons over a wide range of the visible and infrared spectrum for photocatalytic, optoelectronic, and photon conversion applications. Energy transfer from the perovskite nanocrystal to an acceptor dye in the form of a triplet or singlet state offers additional opportunities to tune the properties of the semiconductor–dye hybrid and extend excited-state lifetimes. We have now successfully established the key factors that dictate triplet energy transfer between excited CsPbI3 and surface-bound rhodamine dyes using absorption and emission spectroscopies. The pendant groups on the acceptor dyes influence surface binding to the nanocrystals, which in turn dictate the energy transfer kinetics, as well as the efficiency of energy transfer. Of the three rhodamine dyes investigated (rhodamine B, rhodamine B isothiocyanate, and rose Bengal), the CsPbI3–rose Bengal hybrid with the strongest binding showed the highest triplet energy transfer efficiency (96%) with a rate constant of 1 × 109 s-1. This triplet energy transfer rate constant is nearly 2 orders of magnitude slower than the singlet energy transfer observed for the pure-bromide CsPbBr3–rose Bengal hybrid (1.1 × 1011 s-1). Intriguingly, although the single-halide CsPbBr3 and CsPbI3 nanocrystals selectively populate singlet and triplet excited states of rose Bengal, respectively, the mixed halide perovskites were able to generate a mixture of both singlet and triplet excited states. By tuning the bromide/iodide ratio and thus bandgap energy in CsPb(Br1-xIx)3 compositions, the percentage of singlets vs triplets delivered to the acceptor dye was systematically tuned from 0 to 100%. The excited-state properties of halide perovskite-molecular hybrids discussed here provide new ways to modulate singlet and triplet energy transfer in semiconductor–molecular dye hybrids through acceptor functionalization and donor bandgap engineering.
 Photocatalytic membranes prepared with semiconductor nanoparticles embedded in a polymer film offer a convenient approach to direct the electron and hole flow and separate reduction and oxidation products. We have now embedded In2S3 and ZnS semiconductor nanoparticles in a Nafion membrane to induce photocatalytic reactions using visible light. In addition, we incorporated a viologen redox relay within the membrane to facilitate electron transfer to thionine (TH) dissolved in water. By inserting the photocatalytic membrane in a H-cell, we can separate the oxidation and reduction products and track the electron flow using steady-state photolysis and transient absorption spectroscopy. The enhanced charge separation in the In2S3 and ZnS heterostructure at 50:50 loading allowed us to maximize the electron-transfer yield. Directing such vectorial charge transfer in a photocatalytic membrane will be useful in suppressing undesired side reactions (e.g., re-oxidation of a reduced product) and facilitating product separation.
Photocatalytic membranes prepared with semiconductor nanoparticles embedded in a polymer film offer a convenient approach to direct the electron and hole flow and separate reduction and oxidation products. We have now embedded In2S3 and ZnS semiconductor nanoparticles in a Nafion membrane to induce photocatalytic reactions using visible light. In addition, we incorporated a viologen redox relay within the membrane to facilitate electron transfer to thionine (TH) dissolved in water. By inserting the photocatalytic membrane in a H-cell, we can separate the oxidation and reduction products and track the electron flow using steady-state photolysis and transient absorption spectroscopy. The enhanced charge separation in the In2S3 and ZnS heterostructure at 50:50 loading allowed us to maximize the electron-transfer yield. Directing such vectorial charge transfer in a photocatalytic membrane will be useful in suppressing undesired side reactions (e.g., re-oxidation of a reduced product) and facilitating product separation.
 Directing energy flow in light harvesting assemblies of nanocrystal–chromophore hybrid systems requires a better understanding of factors that dictate excited-state processes. In this study, we explore excited-state interactions within the CsPbI3–cyanine dye (IR125) hybrid assembly through a comprehensive set of steady-state and time-resolved absorption and photoluminescence (PL) experiments. Our photoluminescence investigations reveal the quenching of CsPbI3 emission alongside the simultaneous enhancement of IR125 fluorescence, providing evidence for a singlet energy transfer. The evaluation of both photoluminescence (PL) quenching and PL decay measurements yield ∼94% energy transfer efficiency for the CsPbI3–IR125 hybrid assembly. Transient absorption spectroscopy further unveils that this singlet energy transfer process operates on an ultrafast time scale, occurring within 400 ps with a rate constant of energy transfer of 1.4 × 1010 s-1. Our findings highlight the potential of the CsPbI3–IR125 hybrid assembly to extend the emission of halide perovskites into the infrared region, paving the way for light energy harvesting and display applications.
Directing energy flow in light harvesting assemblies of nanocrystal–chromophore hybrid systems requires a better understanding of factors that dictate excited-state processes. In this study, we explore excited-state interactions within the CsPbI3–cyanine dye (IR125) hybrid assembly through a comprehensive set of steady-state and time-resolved absorption and photoluminescence (PL) experiments. Our photoluminescence investigations reveal the quenching of CsPbI3 emission alongside the simultaneous enhancement of IR125 fluorescence, providing evidence for a singlet energy transfer. The evaluation of both photoluminescence (PL) quenching and PL decay measurements yield ∼94% energy transfer efficiency for the CsPbI3–IR125 hybrid assembly. Transient absorption spectroscopy further unveils that this singlet energy transfer process operates on an ultrafast time scale, occurring within 400 ps with a rate constant of energy transfer of 1.4 × 1010 s-1. Our findings highlight the potential of the CsPbI3–IR125 hybrid assembly to extend the emission of halide perovskites into the infrared region, paving the way for light energy harvesting and display applications.
 Photocatalytic reactions at semiconductor nanocrystal surfaces are useful for synthesizing value-added chemicals using sunlight. Semiconductor nanocrystals dispersed in a rigid framework, such as polymer film, can mitigate issues such as aggregation, product separation, and other challenges that are usually encountered in suspensions or slurries. Using a cation exchange technique, we successfully embedded AgInS2 nanoparticles into a Nafion matrix, termed AgInS2–Nafion. This was achieved through a galvanic exchange between In and Ag in In2S3 present within the Nafion film, enabling an adjustable Ag:In ratio for optimized photophysical properties. As in the case of colloidal suspension, the AgInS2 particles embedded in Nafion exhibit a long absorption tail, a broad emission band with a large Stokes shift, and emission lifetimes extending into the microseconds that are characteristic of donor–acceptor pairs, DAP. Remediation of surface states with the treatment of 3-mercaptopropionic acid resulted in significant enhancement in the emission yield. Charge carrier generation through bandgap excitation as well as activation of DAP states which reside within the bandgap is probed through transient absorption spectroscopy. The photocatalytic activity of AgInS2–Nafion was probed by using thionine as an electron acceptor. The electron transfer rate constant from excited AgInS2 to thionine as observed from transient absorption spectroscopy was determined to be ∼6.3 × 1010 s-1. The design of a photoactive membrane offers new ways to carry out photocatalytic processes with greater selectivity.
Photocatalytic reactions at semiconductor nanocrystal surfaces are useful for synthesizing value-added chemicals using sunlight. Semiconductor nanocrystals dispersed in a rigid framework, such as polymer film, can mitigate issues such as aggregation, product separation, and other challenges that are usually encountered in suspensions or slurries. Using a cation exchange technique, we successfully embedded AgInS2 nanoparticles into a Nafion matrix, termed AgInS2–Nafion. This was achieved through a galvanic exchange between In and Ag in In2S3 present within the Nafion film, enabling an adjustable Ag:In ratio for optimized photophysical properties. As in the case of colloidal suspension, the AgInS2 particles embedded in Nafion exhibit a long absorption tail, a broad emission band with a large Stokes shift, and emission lifetimes extending into the microseconds that are characteristic of donor–acceptor pairs, DAP. Remediation of surface states with the treatment of 3-mercaptopropionic acid resulted in significant enhancement in the emission yield. Charge carrier generation through bandgap excitation as well as activation of DAP states which reside within the bandgap is probed through transient absorption spectroscopy. The photocatalytic activity of AgInS2–Nafion was probed by using thionine as an electron acceptor. The electron transfer rate constant from excited AgInS2 to thionine as observed from transient absorption spectroscopy was determined to be ∼6.3 × 1010 s-1. The design of a photoactive membrane offers new ways to carry out photocatalytic processes with greater selectivity.
 Perovskite solar cells with 2D/3D architecture are claimed to exhibit better stability compared to pristine 3D films at room temperature. However, under illumination and/or heat, cation migration causes the exchange of the bulky spacer cations (2D phase) with the smaller A-site cations (3D phase), creating a gradient heterostructure at the 2D/3D interface. We have evaluated the performance of BA2MAPb2I7/MAPbI3 (2D/3D) and MAPbI3 (3D) solar cells at different temperatures, while simultaneously probing the absorption changes of the 2D/3D perovskite layer and the photovoltaic performance of solar cell devices. The 2D/3D solar cells were more stable at room temperature but exhibited deterioration of photovoltaic performance at high temperatures. By employing in situ measurements of operating solar cells to track both the photoconversion efficiency and absorption changes at different temperatures, we show that the cation exchange at the 2D/3D interface contributes to the efficiency losses.
Perovskite solar cells with 2D/3D architecture are claimed to exhibit better stability compared to pristine 3D films at room temperature. However, under illumination and/or heat, cation migration causes the exchange of the bulky spacer cations (2D phase) with the smaller A-site cations (3D phase), creating a gradient heterostructure at the 2D/3D interface. We have evaluated the performance of BA2MAPb2I7/MAPbI3 (2D/3D) and MAPbI3 (3D) solar cells at different temperatures, while simultaneously probing the absorption changes of the 2D/3D perovskite layer and the photovoltaic performance of solar cell devices. The 2D/3D solar cells were more stable at room temperature but exhibited deterioration of photovoltaic performance at high temperatures. By employing in situ measurements of operating solar cells to track both the photoconversion efficiency and absorption changes at different temperatures, we show that the cation exchange at the 2D/3D interface contributes to the efficiency losses.
 2D/3D interfaces provide long-term stability for the operation of metal halide perovskite solar cells. However, the cation migration under heat and light can disturb the interface and create a graded cation interface. This study has now probed the cation migration by physically pairing X2PbI4 (X = butylammonium BA, oleylammonium OA, or phenethylammonium PEA) 2D film and (CH3NH3, MA)PbI3 3D film at different temperatures and recording changes in the absorption and emission spectra. The migration of the methyl ammonium cation toward the 2D film slowly transforms the n = 1 layered phase into n = 2 and 3 layered phases. The 3D film, on the other hand, exhibits relatively small changes, as the inclusion of spacer cation has little effect on its phase. The apparent activation energy determined from the temperature-dependent cation migration kinetics (Ea = 29– 50 kJ mol-1) indicates alkyl ammonium cations such as butyl ammonium migrate more readily into the 3D layer than aromatic cations such as PEA. The ease of migration of spacer cations between physically paired films suggests that the varied composition at the interface should be considered while evaluating the effectiveness of the 2D/3D design strategy for improving the performance of perovskite solar cells.
2D/3D interfaces provide long-term stability for the operation of metal halide perovskite solar cells. However, the cation migration under heat and light can disturb the interface and create a graded cation interface. This study has now probed the cation migration by physically pairing X2PbI4 (X = butylammonium BA, oleylammonium OA, or phenethylammonium PEA) 2D film and (CH3NH3, MA)PbI3 3D film at different temperatures and recording changes in the absorption and emission spectra. The migration of the methyl ammonium cation toward the 2D film slowly transforms the n = 1 layered phase into n = 2 and 3 layered phases. The 3D film, on the other hand, exhibits relatively small changes, as the inclusion of spacer cation has little effect on its phase. The apparent activation energy determined from the temperature-dependent cation migration kinetics (Ea = 29– 50 kJ mol-1) indicates alkyl ammonium cations such as butyl ammonium migrate more readily into the 3D layer than aromatic cations such as PEA. The ease of migration of spacer cations between physically paired films suggests that the varied composition at the interface should be considered while evaluating the effectiveness of the 2D/3D design strategy for improving the performance of perovskite solar cells.
 Provided is a comprehensive description of a band gap thermodynamic model, which predicts and explains key features of photosegregation in lead-based, mixed-halide perovskites. The model gives a prescription for illustrating halide migration driven by photocarrier energies. Where possible, model predictions are compared with experimental results. Free energy derivations are provided for three assumptions: (1) halide mixing in the dark, (2) a fixed number of photogenerated carriers funneling to and localizing in low band gap inclusions of the alloy, and (3) the statistical occupancy of said inclusions from a bath of thermalized photocarriers in the parent material. Model predictions include excitation intensity (Iexc)-dependent terminal halide stoichiometries (xterminal), temperature-independent xterminal, excitation intensity thresholds (Iexc,threshold) below which photosegregation is suppressed, and reduced segregation in nanocrystals as compared to thin films. The model also offers insight into kinetically manipulating photosegregation rates via control of underlying mediators and rationalizes asymmetries in forward and reverse photosegregation rate constants/activation energies. What emerges is a cohesive framework for understanding ubiquitous photosegregation in mixed-halide perovskites and a rational basis by which to manage the phenomenon.
Provided is a comprehensive description of a band gap thermodynamic model, which predicts and explains key features of photosegregation in lead-based, mixed-halide perovskites. The model gives a prescription for illustrating halide migration driven by photocarrier energies. Where possible, model predictions are compared with experimental results. Free energy derivations are provided for three assumptions: (1) halide mixing in the dark, (2) a fixed number of photogenerated carriers funneling to and localizing in low band gap inclusions of the alloy, and (3) the statistical occupancy of said inclusions from a bath of thermalized photocarriers in the parent material. Model predictions include excitation intensity (Iexc)-dependent terminal halide stoichiometries (xterminal), temperature-independent xterminal, excitation intensity thresholds (Iexc,threshold) below which photosegregation is suppressed, and reduced segregation in nanocrystals as compared to thin films. The model also offers insight into kinetically manipulating photosegregation rates via control of underlying mediators and rationalizes asymmetries in forward and reverse photosegregation rate constants/activation energies. What emerges is a cohesive framework for understanding ubiquitous photosegregation in mixed-halide perovskites and a rational basis by which to manage the phenomenon.
 Energy transfer between a sensitizer and a triplet annihilator-emitter system is a key process that dictates the design of a light energy upconversion system. Using a combination of steady-state and time-resolved emission experiments, we have now elucidated excited-state interactions between the rubrene dye and a perylene derivative (DBP), which are commonly employed in upconversion molecular assemblies. Quenching of rubrene emission with a concomitant increase in DBP emission in rubrene-DBP films confirms singlet-energy transfer with a singlet energy transfer efficiency as high as 94%. Under low-intensity (steady-state) excitation, the singlet excited state of rubrene acts as a primary donor in the Förster resonance energy transfer process. The rate constant of energy transfer as determined from emission lifetime measurements of rubrene-DBP films was 1.4 × 109 s-1. The factors that control the singlet energy transfer between excited rubrene and DBP and its role in designing light energy harvesting assemblies are discussed.
Energy transfer between a sensitizer and a triplet annihilator-emitter system is a key process that dictates the design of a light energy upconversion system. Using a combination of steady-state and time-resolved emission experiments, we have now elucidated excited-state interactions between the rubrene dye and a perylene derivative (DBP), which are commonly employed in upconversion molecular assemblies. Quenching of rubrene emission with a concomitant increase in DBP emission in rubrene-DBP films confirms singlet-energy transfer with a singlet energy transfer efficiency as high as 94%. Under low-intensity (steady-state) excitation, the singlet excited state of rubrene acts as a primary donor in the Förster resonance energy transfer process. The rate constant of energy transfer as determined from emission lifetime measurements of rubrene-DBP films was 1.4 × 109 s-1. The factors that control the singlet energy transfer between excited rubrene and DBP and its role in designing light energy harvesting assemblies are discussed.
 The photostability of lead halide perovskites is an important criterion for their utilization in light harvesting applications. Colloidal two-dimensional mixed halide perovskites offer a convenient approach to probe photoinduced transformations using spectroscopy tools. We employed suspensions of colloidal 2D methylammonium lead halide perovskite platelets with butylammonium and octylammonium as organic spacer cations. Upon irradiation of the mixed halide perovskite (BAOAMAPb2(Br3.5I3.5)) colloidal platelet suspension, we observe a phase transformation, as seen from the formation of bromide rich domains (BAOAMAPb2Br7) and an iodide complex (PbI3–). Unlike in the case of 3D mixed halide films, we did not observe the formation of iodide rich domains as part of the photoinduced phase segregation process. Instead, we observed the dissociation of the BAOAMAPb2I7 to form an iodide complex, PbI3–. Interestingly, this photoinduced phase transformation was reversible, as the products which were confined in the colloidal core restored the original mixed halide phase upon storage in dark. The spectroscopic measurements that characterize excited states of different colloidal methylammonium lead halide perovskites and kinetics of photoinduced transformation and their dark recovery are presented. These new phototransformations of colloidal perovskite platelets provide new insights into the processes that dictate their overall photostability.
The photostability of lead halide perovskites is an important criterion for their utilization in light harvesting applications. Colloidal two-dimensional mixed halide perovskites offer a convenient approach to probe photoinduced transformations using spectroscopy tools. We employed suspensions of colloidal 2D methylammonium lead halide perovskite platelets with butylammonium and octylammonium as organic spacer cations. Upon irradiation of the mixed halide perovskite (BAOAMAPb2(Br3.5I3.5)) colloidal platelet suspension, we observe a phase transformation, as seen from the formation of bromide rich domains (BAOAMAPb2Br7) and an iodide complex (PbI3–). Unlike in the case of 3D mixed halide films, we did not observe the formation of iodide rich domains as part of the photoinduced phase segregation process. Instead, we observed the dissociation of the BAOAMAPb2I7 to form an iodide complex, PbI3–. Interestingly, this photoinduced phase transformation was reversible, as the products which were confined in the colloidal core restored the original mixed halide phase upon storage in dark. The spectroscopic measurements that characterize excited states of different colloidal methylammonium lead halide perovskites and kinetics of photoinduced transformation and their dark recovery are presented. These new phototransformations of colloidal perovskite platelets provide new insights into the processes that dictate their overall photostability.
 Movement of energy within light-harvesting assemblies is typically carried out with separately synthesized donor and acceptor species, which are then brought together to induce an interaction. Recently, two-dimensional (2D) lead halide perovskites have gained interest for their ability to accommodate and assemble chromophoric molecules within their lattice, creating hybrid organic–inorganic compositions. Using a combination of steady-state and time-resolved absorption and emission spectroscopy, we have now succeeded in establishing the competition between energy transfer and charge trapping in 2D halide perovskite colloids containing naphthalene-derived cations (i.e., NEA2PbX4, where NEA = naphthylethylamine). The presence of room-temperature triplet emission from the naphthalene moiety depends on the ratio of bromide to iodide in the lead halide sublattice (i.e., x in NEA2Pb(Br1–xIx)4), with only bromide-rich compositions showing sensitized emission. Photoluminescence lifetime measurements of the sensitized naphthalene reveal the formation of the naphthalene triplet excimer at room temperature. From transient absorption measurements, we find the rate constant of triplet energy transfer (kEnT) to be on the order of ∼109 s–1. At low temperatures (77 K) a new broad emission feature arising from trap states is observed in all samples ranging from pure bromide to pure iodide composition. These results reveal the interplay between sensitized triplet energy transfer and charge trapping in 2D lead halide perovskites, highlighting the need to carefully parse contributions from competing de-excitation pathways for optoelectronic applications.
Movement of energy within light-harvesting assemblies is typically carried out with separately synthesized donor and acceptor species, which are then brought together to induce an interaction. Recently, two-dimensional (2D) lead halide perovskites have gained interest for their ability to accommodate and assemble chromophoric molecules within their lattice, creating hybrid organic–inorganic compositions. Using a combination of steady-state and time-resolved absorption and emission spectroscopy, we have now succeeded in establishing the competition between energy transfer and charge trapping in 2D halide perovskite colloids containing naphthalene-derived cations (i.e., NEA2PbX4, where NEA = naphthylethylamine). The presence of room-temperature triplet emission from the naphthalene moiety depends on the ratio of bromide to iodide in the lead halide sublattice (i.e., x in NEA2Pb(Br1–xIx)4), with only bromide-rich compositions showing sensitized emission. Photoluminescence lifetime measurements of the sensitized naphthalene reveal the formation of the naphthalene triplet excimer at room temperature. From transient absorption measurements, we find the rate constant of triplet energy transfer (kEnT) to be on the order of ∼109 s–1. At low temperatures (77 K) a new broad emission feature arising from trap states is observed in all samples ranging from pure bromide to pure iodide composition. These results reveal the interplay between sensitized triplet energy transfer and charge trapping in 2D lead halide perovskites, highlighting the need to carefully parse contributions from competing de-excitation pathways for optoelectronic applications.
 In-situ polymer capping of cesium lead bromide (CsPbBr3) nanocrystals with polymethyl acrylate is an effective approach to improve the colloidal stability in the polar medium and thus extends their use in photocatalysis. The photoinduced electron transfer properties of polymethyl acrylate (PMA)-capped CsPbBr3 nanocrystals have been probed using surface-bound viologen molecules with different alkyl chains as electron acceptors. The apparent association constant (Kapp) obtained for the binding of viologen molecules with PMA-capped CsPbBr3 was 2.3 × 107 M-1, which is an order of magnitude greater than that obtained with oleic acid/oleylamine-capped CsPbBr3. Although the length of the alkyl chain of the viologen molecule did not show any impact on the electron transfer rate constant, it influenced the charge separation efficiency and net electron transfer quantum yield. Viologen moieties with a shorter alkyl chain length exhibited a charge separation efficiency of 72% compared with 50% for the longer chain alkyl chain length viologens. Implications of polymer-capped CsPbBr3 perovskite nanocrystals for carrying out photocatalytic reduction in the polar medium are discussed.
In-situ polymer capping of cesium lead bromide (CsPbBr3) nanocrystals with polymethyl acrylate is an effective approach to improve the colloidal stability in the polar medium and thus extends their use in photocatalysis. The photoinduced electron transfer properties of polymethyl acrylate (PMA)-capped CsPbBr3 nanocrystals have been probed using surface-bound viologen molecules with different alkyl chains as electron acceptors. The apparent association constant (Kapp) obtained for the binding of viologen molecules with PMA-capped CsPbBr3 was 2.3 × 107 M-1, which is an order of magnitude greater than that obtained with oleic acid/oleylamine-capped CsPbBr3. Although the length of the alkyl chain of the viologen molecule did not show any impact on the electron transfer rate constant, it influenced the charge separation efficiency and net electron transfer quantum yield. Viologen moieties with a shorter alkyl chain length exhibited a charge separation efficiency of 72% compared with 50% for the longer chain alkyl chain length viologens. Implications of polymer-capped CsPbBr3 perovskite nanocrystals for carrying out photocatalytic reduction in the polar medium are discussed.
 Energy and electron transfer processes allow for efficient manipulation of excited states within light harvesting assemblies for photocatalytic and optoelectronic applications. We have now successfully probed the influence of acceptor pendant group functionalization on the energy and electron transfer between CsPbBr3 perovskite nanocrystals and three rhodamine-based acceptor molecules. The three acceptors─rhodamine B (RhB), rhodamine isothiocyanate (RhB-NCS), and rose Bengal (RoseB)─contain an increasing degree of pendant group functionalization that affects their native excited state properties. When interacting with CsPbBr3 as an energy donor, photoluminescence excitation spectroscopy reveals that singlet energy transfer occurs with all three acceptors. However, the acceptor functionalization directly influences several key parameters that dictate the excited state interactions. For example, RoseB binds to the nanocrystal surface with an apparent association constant (Kapp = 9.4 × 106 M-1) 200 times greater than RhB (Kapp = 0.05 × 106 M-1), thus influencing the rate of energy transfer. Femtosecond transient absorption reveals the observed rate constant of singlet energy transfer (kEnT) is an order-of-magnitude greater for RoseB (kEnT = 1 × 1011 s-1) than for RhB and RhB-NCS. In addition to energy transfer, each acceptor had a subpopulation of molecules (∼30%) that underwent electron transfer as a competing pathway. Thus, the structural influence of acceptor moieties must be considered for both excited state energy and electron transfer in nanocrystal-molecular hybrids. The competition between electron and energy transfer further highlights the complexity of excited state interactions in nanocrystal-molecular complexes and the need for careful spectroscopic analysis to elucidate competitive pathways.
Energy and electron transfer processes allow for efficient manipulation of excited states within light harvesting assemblies for photocatalytic and optoelectronic applications. We have now successfully probed the influence of acceptor pendant group functionalization on the energy and electron transfer between CsPbBr3 perovskite nanocrystals and three rhodamine-based acceptor molecules. The three acceptors─rhodamine B (RhB), rhodamine isothiocyanate (RhB-NCS), and rose Bengal (RoseB)─contain an increasing degree of pendant group functionalization that affects their native excited state properties. When interacting with CsPbBr3 as an energy donor, photoluminescence excitation spectroscopy reveals that singlet energy transfer occurs with all three acceptors. However, the acceptor functionalization directly influences several key parameters that dictate the excited state interactions. For example, RoseB binds to the nanocrystal surface with an apparent association constant (Kapp = 9.4 × 106 M-1) 200 times greater than RhB (Kapp = 0.05 × 106 M-1), thus influencing the rate of energy transfer. Femtosecond transient absorption reveals the observed rate constant of singlet energy transfer (kEnT) is an order-of-magnitude greater for RoseB (kEnT = 1 × 1011 s-1) than for RhB and RhB-NCS. In addition to energy transfer, each acceptor had a subpopulation of molecules (∼30%) that underwent electron transfer as a competing pathway. Thus, the structural influence of acceptor moieties must be considered for both excited state energy and electron transfer in nanocrystal-molecular hybrids. The competition between electron and energy transfer further highlights the complexity of excited state interactions in nanocrystal-molecular complexes and the need for careful spectroscopic analysis to elucidate competitive pathways.
 A (PEA)2PbI4 (2D) layer deposited on a MAPbI3 (3D) film undergoes a transformation upon photoirradiation and thermal treatment. The decrease in absorption and emission of the 2D layer during light and heat exposure, which shows migration of the PEA+ cation within the 3D film, points out the dynamic nature of the 2D/3D interface and intermixing of the two phases.
A (PEA)2PbI4 (2D) layer deposited on a MAPbI3 (3D) film undergoes a transformation upon photoirradiation and thermal treatment. The decrease in absorption and emission of the 2D layer during light and heat exposure, which shows migration of the PEA+ cation within the 3D film, points out the dynamic nature of the 2D/3D interface and intermixing of the two phases.
 Transient chemistry of sensitizing dyes is important to obtain insights into the photochemical conversion processes of light harvesting assemblies. We have now employed transient absorption spectroscopy (pulsed laser and pulse radiolysis) to characterize the excited state and radical intermediates of a perylene derivative, (5,10,15,20-Tetraphenylbisbenz[5,6]indeno[1,2,3-cd:1′,2′,3′-lm]perylene (DBP). The distinguishable transient absorption features for the singlet and triplet excited states and radical anion and radical cation provide spectral fingerprints to identify the reaction intermediates in photochemical energy and electron transfer processes of composite systems involving DBP. For example, identifying these transients in the energy transfer processes of the rubrene–DBP system would aid in establishing their role as annihilator-emitter for triplet–triplet annihilation up-conversion (TTA-UC). The transient characterization thus serves as an important mechanistic fingerprint for elucidating mechanistic details of systems employing DBP in optoelectronic applications.
Transient chemistry of sensitizing dyes is important to obtain insights into the photochemical conversion processes of light harvesting assemblies. We have now employed transient absorption spectroscopy (pulsed laser and pulse radiolysis) to characterize the excited state and radical intermediates of a perylene derivative, (5,10,15,20-Tetraphenylbisbenz[5,6]indeno[1,2,3-cd:1′,2′,3′-lm]perylene (DBP). The distinguishable transient absorption features for the singlet and triplet excited states and radical anion and radical cation provide spectral fingerprints to identify the reaction intermediates in photochemical energy and electron transfer processes of composite systems involving DBP. For example, identifying these transients in the energy transfer processes of the rubrene–DBP system would aid in establishing their role as annihilator-emitter for triplet–triplet annihilation up-conversion (TTA-UC). The transient characterization thus serves as an important mechanistic fingerprint for elucidating mechanistic details of systems employing DBP in optoelectronic applications.
 Photoelectrochemical (PEC) water splitting converts solar light and water into oxygen and energy-rich hydrogen. WO3/BiVO4 heterojunction photoanodes perform much better than the separate oxide components, though internal charge recombination undermines their PEC performance when both oxides absorb light. Here we exploit the BiVO4 layer to sensitize WO4 to visible light and shield it from direct photoexcitation to overcome this efficiency loss. PEC experiments and ultrafast transient absorption spectroscopy performed by frontside (through BiVO4) or backside (through WO3) irradiating photoanodes with different BiVO4 layer thickness demonstrate that irradiation through BiVO4 is beneficial for charge separation. Optimized electrodes irradiated through BiVO4 show 40% higher photocurrent density compared to backside irradiation.
Photoelectrochemical (PEC) water splitting converts solar light and water into oxygen and energy-rich hydrogen. WO3/BiVO4 heterojunction photoanodes perform much better than the separate oxide components, though internal charge recombination undermines their PEC performance when both oxides absorb light. Here we exploit the BiVO4 layer to sensitize WO4 to visible light and shield it from direct photoexcitation to overcome this efficiency loss. PEC experiments and ultrafast transient absorption spectroscopy performed by frontside (through BiVO4) or backside (through WO3) irradiating photoanodes with different BiVO4 layer thickness demonstrate that irradiation through BiVO4 is beneficial for charge separation. Optimized electrodes irradiated through BiVO4 show 40% higher photocurrent density compared to backside irradiation.
 Two-dimensional (2D) Ruddlesden–Popper mixed-halide perovskite films, BA2PbBr2I2, undergo phase segregation when excited with visible light to generate bromide- and iodide-rich regions, as marked by absorption and emission changes. Upon stopping illumination, the process reverses, allowing original film compositions to be restored. However, if films are in contact with dichloromethane, light irradiation causes the sequential expulsion of iodide and bromide and introduces irreversible changes to the 2D films. The sequential disappearance of I- and Br- from pristine films (BA2PbBr4 and BA2PbI4) under photoirradiation, as observed from variances in expulsion rates, reflects differences in halide ion mobilities in these films. The photoinstability of 2D films raises questions about their use in stabilizing bulk, three-dimensional (3D) perovskite solar cells through 3D/2D interfaces.
Two-dimensional (2D) Ruddlesden–Popper mixed-halide perovskite films, BA2PbBr2I2, undergo phase segregation when excited with visible light to generate bromide- and iodide-rich regions, as marked by absorption and emission changes. Upon stopping illumination, the process reverses, allowing original film compositions to be restored. However, if films are in contact with dichloromethane, light irradiation causes the sequential expulsion of iodide and bromide and introduces irreversible changes to the 2D films. The sequential disappearance of I- and Br- from pristine films (BA2PbBr4 and BA2PbI4) under photoirradiation, as observed from variances in expulsion rates, reflects differences in halide ion mobilities in these films. The photoinstability of 2D films raises questions about their use in stabilizing bulk, three-dimensional (3D) perovskite solar cells through 3D/2D interfaces.