Photocatalysis
Background
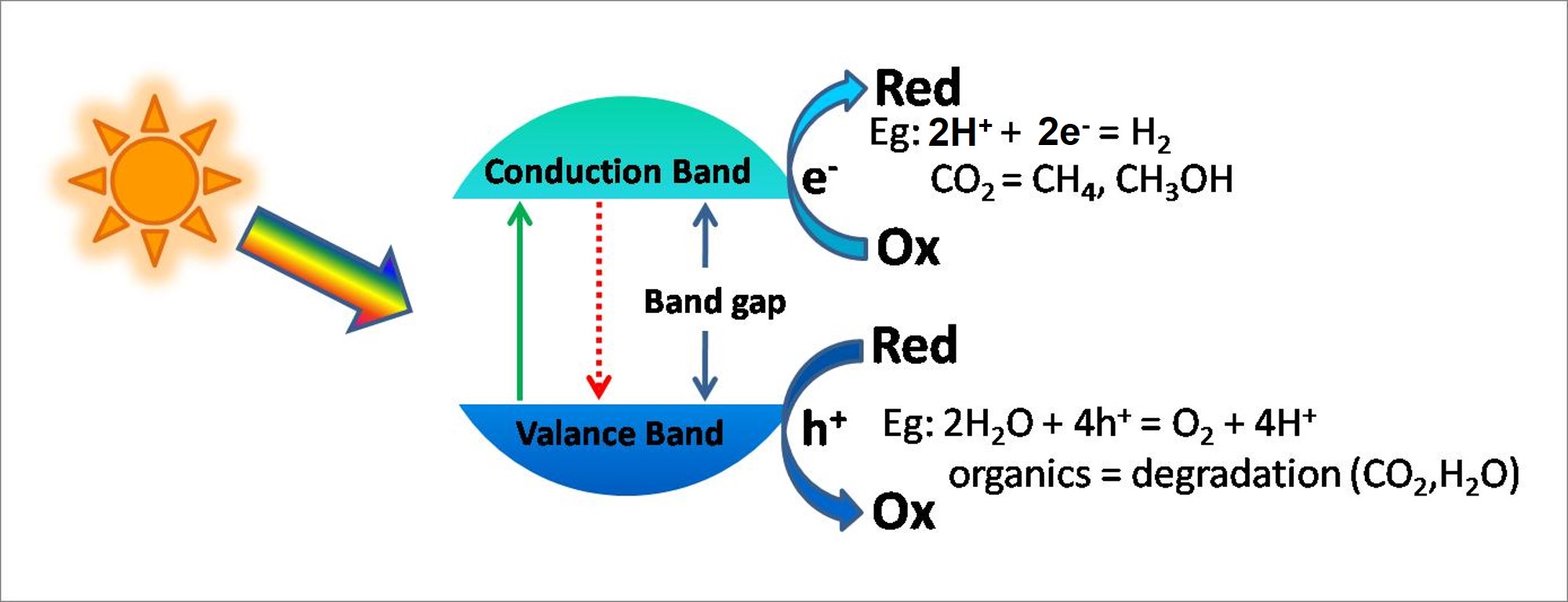 Semiconductors are useful candidate for photocatalysis, solar energy can be used to carry out chemical reactions including H2 fuel generation from water.
Semiconductors are useful candidate for photocatalysis, solar energy can be used to carry out chemical reactions including H2 fuel generation from water.
Semiconductor photocatalysts are semiconductors which are able cause and/or accelerate chemical reactions upon light absorption, typically sunlight. By utilizing the energy of absorbed photons, photocatalysts can be optimized carry out a wide variety of important chemical processes such as
- Environmental remediation - the destruction of organic pollutants for water or air purification
- Solar Fuels Production - production of fuels, H2 from water or methane/methanol from CO2
Semiconductors can be used in photocatalysis as particle dispersions or in photoelectrochemical mode. Several hybrid structures have been envisaged to improve photocatalytic performance of semiconductors such as semiconductor-semiconductor, semiconductor-metal, semiconductor-Reduced Graphene Oxide (RGO) nano-assemblies. It is important we understand the intricate interface electron dynamics in all of these assemblies which will allow us to use these systems in various applications. Our research interests include understanding the behaviour of the above nano composited used for photocatalysis.
Photocatalytic Properties of TiO2
Titanium dioxide (TiO2) is a semiconductor with a band gap of 3.2 eV. It is an abundant material with wide-ranging uses, and it is known to be exceptionally stable in a variety of conditions. TiO2 is frequently used as a whitener in both foods and industrial products like sunscreen or paint. Research has also shown that TiO2 can be used to destroy organic compounds, prevent fogging of glass, and even split water into hydrogen and oxygen.
In our lab, we have done extensive research into understanding both the capabilities and fundamental processes of TiO2. We have explored its ability to decompose molecules and its use as a substrate for dye-sensitized, quantum dot-sensitized, and solid state solar cells. The photocatalytic activity of TiO2 comes from two sources: 1) generation of OH· radicals by oxidation of OH- anions and 2) generation of O2- radicals by reduction of O2. Both of these radical anions can react with other species to degrade or otherwise change them, making TiO2 an effective photocatalyst for many applications. The anatase phase of TiO2 is arguably the most photoactive, and a great portion of the literature focuses on the use of this crystalline phase. Still, the more thermodynamically stable form of TiO2, rutile, has also been the focus of a great deal of work.
- Photocatalysis - Background information and history of photocatalysis
- Uses and Properties of TiO2 - Further information on the photocatalytic properties and the industrial and commercial uses of Titanium Dioxide
Our Research Focus
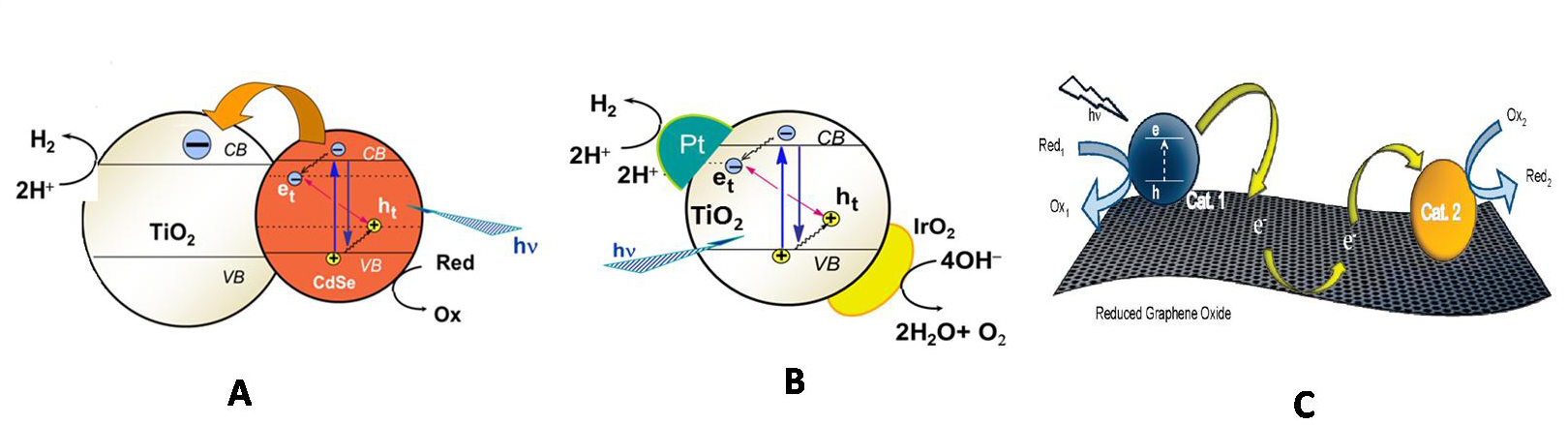
Nano composites for photocatalysis with (A) semiconductor-semiconductor (B) semiconductor -metal (C) semiconductor-RGO hybrid architectures.
- Understand the interfacial charge transfer processes in (A)semiconductor-semiconductor (B) semiconductor -metal (C) semiconductor-RGO hybrid architectures for photocatalysis
- Improve charge separation in the semiconductors using the above mentioned architectures
- Extend the light absorption of photocatalysts into the visible region of the solar spectrum to utilize a greater portion of the incident solar radiation
- Employ capacitative metal nanoparticles (Ag and Au) –semiconductor- RGO nanocomposites for H2 generation
Recent Progress
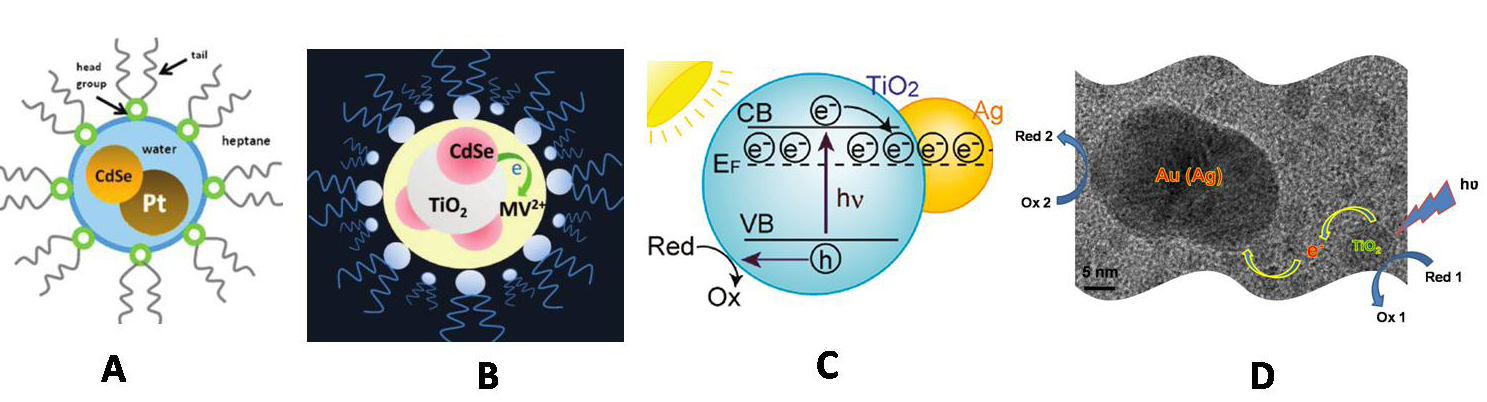
Nano composites for photocatalysis with (A) semiconductor-metal (B) semiconductor - semiconductor in confined media (C) semiconductor-Ag hybrid structures (D) semiconductor-RGO hybrid architectures.
- Pioneered a solution-based method for the formation of "holey" graphene with potential benefits to sensing as well as other fields
- Performed galvanic exchange on the surface of graphene oxide to provide a multifunctional catalyst assembly
- Observed photocatalytic events of CdSe Quantum Dffots in Confined Media
- Demonstraged the storing and shuttling of electrons across RGO surface
Select Publications
439. Making Graphene Holey. Gold-Nanoparticle-Mediated Hydroxyl Radical Attack on Reduced Graphene Oxide Radich, J. G.; Kamat, P. V. ACS Nano 2013, 7 (6), 5546–5557.
432. Galvanic Exchange on Reduced Graphene Oxide. Designing a Multifunctional Two-Dimensional Catalyst Assembly. Krishnamurthy, S.; Kamat, P. V. J. Phys. Chem. C 2013, 117 (1), 571–577.
429. Dual-Frequency Ultrasound for Designing Two Dimensional Catalyst Surface: Reduced Graphene Oxide-Pt Composite. Vinodgopal, K.; Neppolian, B.; Salleh, N.; Lightcap, I. V.; Grieser, F.; Ashokkumar, M.; Ding, T. T.; Kamat, P. V. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2012, 5, 81-87.
421. Manipulation of Charge Transfer Across Semiconductor Interface. A Criterion that Cannot be Ignored in Photocatalyst Design. Kamat, P. V. J.Phys. Chem. Lett. 2012, 3 ASAP. (Perspective article)
419. Photoinduced Charging and Discharging of ZnO Nanoparticles on Graphene Oxide Sheets Yokomizo, Y.; Krishnamurthy, S.; Kamat, P. V.Catalysis Today 2012, submitted.
414. Capture, Store and Discharge. Shuttling Photogenerated Electrons across TiO2-Silver Interface Takai, A.; Kamat, P. V., ACS Nano 2011, 5, 7369–7376.
403. Photocatalytic Events of CdSe Quantum Dots in Confined Media. Electrodic Behavior of Coupled Platinum Nanoparticles Harris, C.; Kamat, P. V. ACS Nano 2010, 4 (12), 7312-7330.
398. To What Extent Do Graphene Scaffolds Improve the Photovoltaic and Photocatalytic Response of TiO2 Nanostructured Films? Ng, Yun Hau, Lightcap, I. V.; Goodwin, K.; Matsumura, M.; Kamat, P. V. J. Phys. Chem. Lett. 2010 1, 2222-2227.
392. Graphene based Nanoarchitectures. Anchoring Semiconductor and Metal Nanoparticles on a 2-Dimensional Carbon Support Kamat, P. V. J. Phys. Chem. Lett. 2010, 1, 520-527. (Perspective)
385. Fuel Cell Geared in Reverse. Photocatalytic Hydrogen Production using a TiO2/Nafion/Pt Membrane Assembly with No Applied Bias Seger, B.; Kamat, P. V. J. Phys. Chem. C 2009, 113,18946–18952. NDRL 4820
377. Photocatalysis with CdSe Nanoparticles in Confined Media: Mapping Charge Transfer Events in the Subpicosecond to Second Timescales Harris, C. T.; Kamat, P. V. ACS Nano 2009, 3, 682-690. NDRL 4790
273. Photophysical, photochemical and photocatalytic aspects of metal nanoparticles. Kamat, P. V. J. Phys. Chem. B 2002, 106, 7729-7744. NDRL 4374 (Feature Article)
