All Publications
2024
582. Energy Cascade in Halide Perovskite-Multiple Chromophore Films: Direct versus Mediated Transfer Jishnudas Chakkamalayath, Lauren E. Martin, and Prashant V. Kamat ACS Photonics 2024, XXXX, XXX, XXX-XXX.
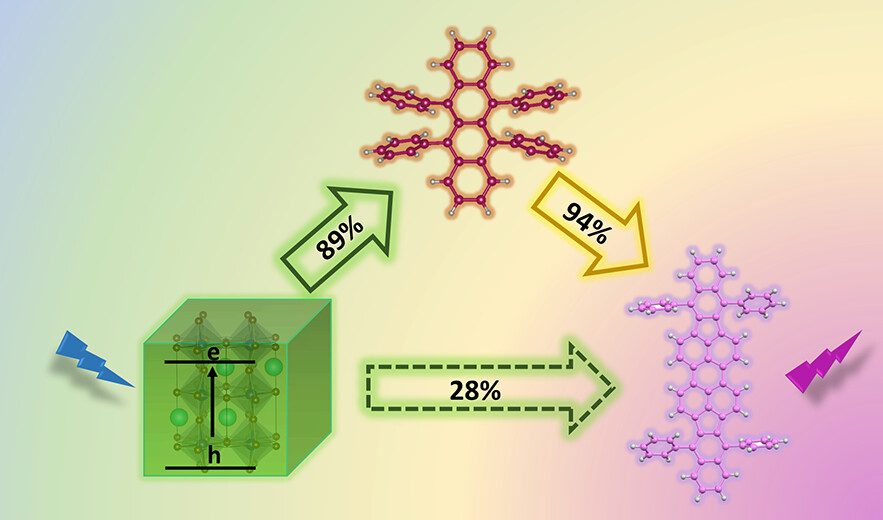 The capability of halide perovskite nanocrystals to sensitize both singlet and triplet excited states of a chromophore highlights their potential as photosensitizers in down conversion (singlet energy transfer) or upconversion (triplet energy transfer followed by triplet–triplet annihilation) applications. In semiconductor-multiple chromophore assemblies, however, various possibilities arise to modulate the energy transfer process and hence the final emission output. By employing CsPbBr3 nanocrystals (Eg = 2.47 eV) as a primary sensitizer, we have now probed the singlet energy transfer to two dyes, viz., rubrene (Es = 2.23 eV) and DBP, a perylene derivative (Es = 2.07 eV). By first characterizing the excited-state interactions between CsPbBr3 and individual dye pairs, we establish the favorable pathway for singlet energy transfer in a donor–acceptor1–acceptor2 assembly. The singlet energy transfer efficiencies for CsPbBr3-rubrene, rubrene-DBP, and CsPbBr3-DBP systems were quantified as 89%, 94%, and 28%, respectively. When all three components (CsPbBr3-rubrene-DBP) are present in the film, we observe a cascading energy transfer to yield a high population of singlet excited-states in DBP. The 1DBP* yield increased with rubrene concentration, thus confirming its mediating role in the energy cascade. Thus, a proper choice of mediator can promote singlet energy transfer when the spectral overlap between a donor and acceptor is poor. Elucidation of the excited-state interactions in CsPbBr3-rubrene DBP films offers new insights into the sensitization of multiple chromophore assemblies and ways to modulate the singlet energy flow.
The capability of halide perovskite nanocrystals to sensitize both singlet and triplet excited states of a chromophore highlights their potential as photosensitizers in down conversion (singlet energy transfer) or upconversion (triplet energy transfer followed by triplet–triplet annihilation) applications. In semiconductor-multiple chromophore assemblies, however, various possibilities arise to modulate the energy transfer process and hence the final emission output. By employing CsPbBr3 nanocrystals (Eg = 2.47 eV) as a primary sensitizer, we have now probed the singlet energy transfer to two dyes, viz., rubrene (Es = 2.23 eV) and DBP, a perylene derivative (Es = 2.07 eV). By first characterizing the excited-state interactions between CsPbBr3 and individual dye pairs, we establish the favorable pathway for singlet energy transfer in a donor–acceptor1–acceptor2 assembly. The singlet energy transfer efficiencies for CsPbBr3-rubrene, rubrene-DBP, and CsPbBr3-DBP systems were quantified as 89%, 94%, and 28%, respectively. When all three components (CsPbBr3-rubrene-DBP) are present in the film, we observe a cascading energy transfer to yield a high population of singlet excited-states in DBP. The 1DBP* yield increased with rubrene concentration, thus confirming its mediating role in the energy cascade. Thus, a proper choice of mediator can promote singlet energy transfer when the spectral overlap between a donor and acceptor is poor. Elucidation of the excited-state interactions in CsPbBr3-rubrene DBP films offers new insights into the sensitization of multiple chromophore assemblies and ways to modulate the singlet energy flow.
581. Ramifications of Ion Migration on 2D Lead Halide Perovskites Preethi Mathew, Junsang Cho, and Prashant V. Kamat ACS Energy Lett. 2024, 9, 3, 1103-1114.
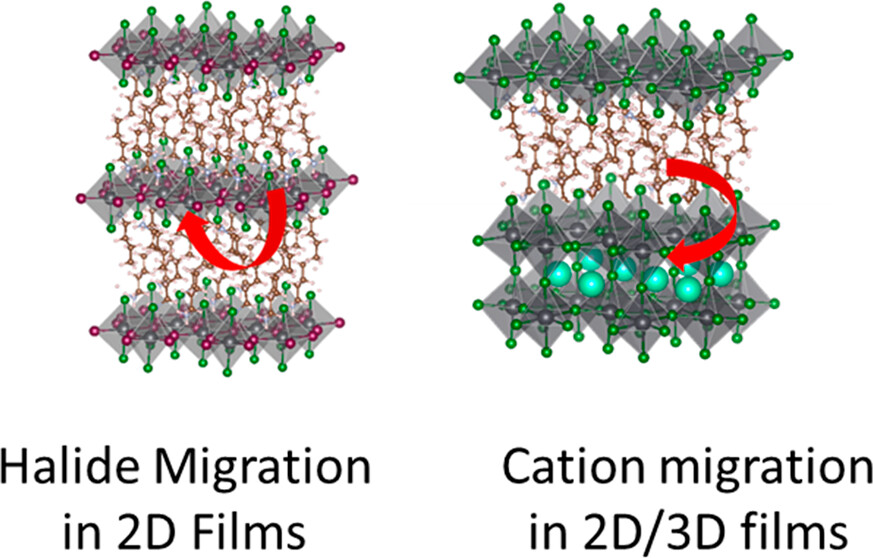 Lower dimensional or 2D halide perovskites, with their versatile structural and functional properties, are known to improve the performance and room temperature stability of perovskite solar cells. One would expect 2D perovskites to be more resistant to ion migration compared to their 3D counterparts because of the presence of bulky organic cations. However, recent findings show that ion migration indeed is prevalent in 2D halide perovskites similar to 3D perovskites. Halide ion migration in 2D perovskites manifests itself in halide ion segregation under photoirradiation as well as in halide exchange between physically paired films of 2D perovskites with different halide ions. Besides halide ion migration, cation migration of spacer cations and A-site cations is also seen when 2D/3D perovskite films are subjected to light and thermal stress. It is important to recognize the importance of ion migration while incorporating 2D perovskites in solar cells and other optoelectronic devices, as it can be detrimental for achieving streamlined performance and long-term stability. This Perspective discusses recent reports on ion migration in 2D and in 2D/3D halide perovskite films under the operational conditions (at elevated temperature and given built-in bias) and presents a few mitigating strategies.
Lower dimensional or 2D halide perovskites, with their versatile structural and functional properties, are known to improve the performance and room temperature stability of perovskite solar cells. One would expect 2D perovskites to be more resistant to ion migration compared to their 3D counterparts because of the presence of bulky organic cations. However, recent findings show that ion migration indeed is prevalent in 2D halide perovskites similar to 3D perovskites. Halide ion migration in 2D perovskites manifests itself in halide ion segregation under photoirradiation as well as in halide exchange between physically paired films of 2D perovskites with different halide ions. Besides halide ion migration, cation migration of spacer cations and A-site cations is also seen when 2D/3D perovskite films are subjected to light and thermal stress. It is important to recognize the importance of ion migration while incorporating 2D perovskites in solar cells and other optoelectronic devices, as it can be detrimental for achieving streamlined performance and long-term stability. This Perspective discusses recent reports on ion migration in 2D and in 2D/3D halide perovskite films under the operational conditions (at elevated temperature and given built-in bias) and presents a few mitigating strategies.
580. Tuning Energy Transfer Pathways in Halide Perovskite–Dye Hybrids through Bandgap Engineering Akshaya Chemmangat, Jishnudas Chakkamalayath, Jeffrey T. DuBose, and Prashant V. Kamat J. Am. Chem. Soc. 2024, 146, 5, 3352-3362.
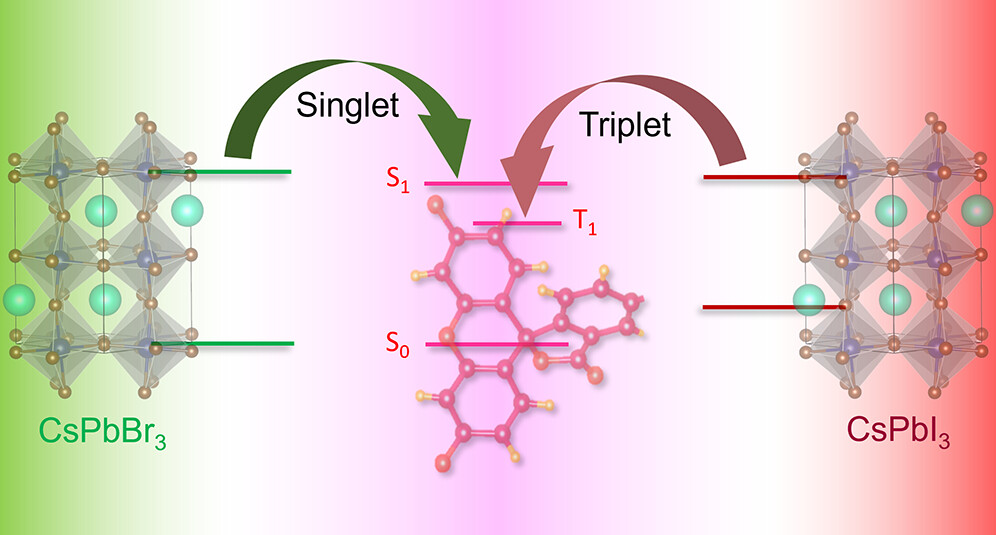 Lead halide perovskite nanocrystals, which offer rich photochemistry, have the potential to capture photons over a wide range of the visible and infrared spectrum for photocatalytic, optoelectronic, and photon conversion applications. Energy transfer from the perovskite nanocrystal to an acceptor dye in the form of a triplet or singlet state offers additional opportunities to tune the properties of the semiconductor–dye hybrid and extend excited-state lifetimes. We have now successfully established the key factors that dictate triplet energy transfer between excited CsPbI3 and surface-bound rhodamine dyes using absorption and emission spectroscopies. The pendant groups on the acceptor dyes influence surface binding to the nanocrystals, which in turn dictate the energy transfer kinetics, as well as the efficiency of energy transfer. Of the three rhodamine dyes investigated (rhodamine B, rhodamine B isothiocyanate, and rose Bengal), the CsPbI3–rose Bengal hybrid with the strongest binding showed the highest triplet energy transfer efficiency (96%) with a rate constant of 1 × 109 s-1. This triplet energy transfer rate constant is nearly 2 orders of magnitude slower than the singlet energy transfer observed for the pure-bromide CsPbBr3–rose Bengal hybrid (1.1 × 1011 s-1). Intriguingly, although the single-halide CsPbBr3 and CsPbI3 nanocrystals selectively populate singlet and triplet excited states of rose Bengal, respectively, the mixed halide perovskites were able to generate a mixture of both singlet and triplet excited states. By tuning the bromide/iodide ratio and thus bandgap energy in CsPb(Br1-xIx)3 compositions, the percentage of singlets vs triplets delivered to the acceptor dye was systematically tuned from 0 to 100%. The excited-state properties of halide perovskite-molecular hybrids discussed here provide new ways to modulate singlet and triplet energy transfer in semiconductor–molecular dye hybrids through acceptor functionalization and donor bandgap engineering.
Lead halide perovskite nanocrystals, which offer rich photochemistry, have the potential to capture photons over a wide range of the visible and infrared spectrum for photocatalytic, optoelectronic, and photon conversion applications. Energy transfer from the perovskite nanocrystal to an acceptor dye in the form of a triplet or singlet state offers additional opportunities to tune the properties of the semiconductor–dye hybrid and extend excited-state lifetimes. We have now successfully established the key factors that dictate triplet energy transfer between excited CsPbI3 and surface-bound rhodamine dyes using absorption and emission spectroscopies. The pendant groups on the acceptor dyes influence surface binding to the nanocrystals, which in turn dictate the energy transfer kinetics, as well as the efficiency of energy transfer. Of the three rhodamine dyes investigated (rhodamine B, rhodamine B isothiocyanate, and rose Bengal), the CsPbI3–rose Bengal hybrid with the strongest binding showed the highest triplet energy transfer efficiency (96%) with a rate constant of 1 × 109 s-1. This triplet energy transfer rate constant is nearly 2 orders of magnitude slower than the singlet energy transfer observed for the pure-bromide CsPbBr3–rose Bengal hybrid (1.1 × 1011 s-1). Intriguingly, although the single-halide CsPbBr3 and CsPbI3 nanocrystals selectively populate singlet and triplet excited states of rose Bengal, respectively, the mixed halide perovskites were able to generate a mixture of both singlet and triplet excited states. By tuning the bromide/iodide ratio and thus bandgap energy in CsPb(Br1-xIx)3 compositions, the percentage of singlets vs triplets delivered to the acceptor dye was systematically tuned from 0 to 100%. The excited-state properties of halide perovskite-molecular hybrids discussed here provide new ways to modulate singlet and triplet energy transfer in semiconductor–molecular dye hybrids through acceptor functionalization and donor bandgap engineering.
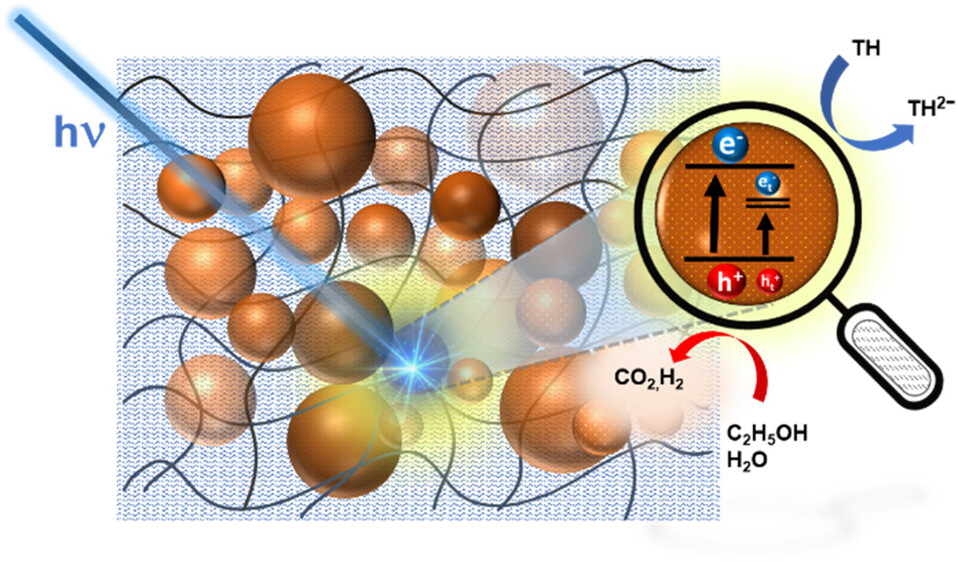
579. Directional Electron Transfer across In2S3/ZnS-Embedded Photocatalytic Membranes Yiseul Yu, Anthony Kipkorir, Myong Yong Choi, and Prashant V. Kamat ACS Appl. Energy Mater. 2024, 7, 2, 681-688.
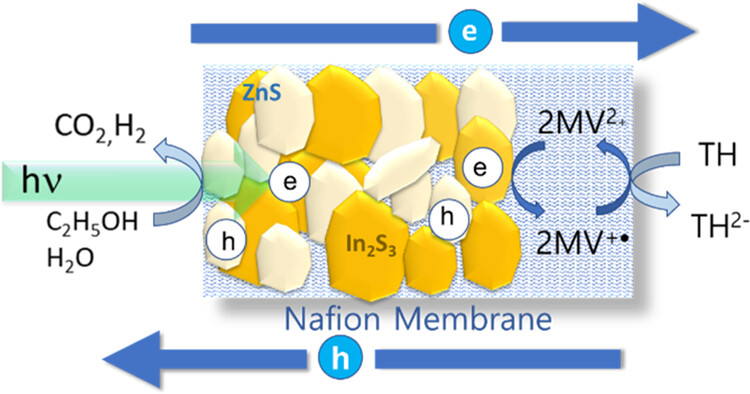 Photocatalytic membranes prepared with semiconductor nanoparticles embedded in a polymer film offer a convenient approach to direct the electron and hole flow and separate reduction and oxidation products. We have now embedded In2S3 and ZnS semiconductor nanoparticles in a Nafion membrane to induce photocatalytic reactions using visible light. In addition, we incorporated a viologen redox relay within the membrane to facilitate electron transfer to thionine (TH) dissolved in water. By inserting the photocatalytic membrane in a H-cell, we can separate the oxidation and reduction products and track the electron flow using steady-state photolysis and transient absorption spectroscopy. The enhanced charge separation in the In2S3 and ZnS heterostructure at 50:50 loading allowed us to maximize the electron-transfer yield. Directing such vectorial charge transfer in a photocatalytic membrane will be useful in suppressing undesired side reactions (e.g., re-oxidation of a reduced product) and facilitating product separation.
Photocatalytic membranes prepared with semiconductor nanoparticles embedded in a polymer film offer a convenient approach to direct the electron and hole flow and separate reduction and oxidation products. We have now embedded In2S3 and ZnS semiconductor nanoparticles in a Nafion membrane to induce photocatalytic reactions using visible light. In addition, we incorporated a viologen redox relay within the membrane to facilitate electron transfer to thionine (TH) dissolved in water. By inserting the photocatalytic membrane in a H-cell, we can separate the oxidation and reduction products and track the electron flow using steady-state photolysis and transient absorption spectroscopy. The enhanced charge separation in the In2S3 and ZnS heterostructure at 50:50 loading allowed us to maximize the electron-transfer yield. Directing such vectorial charge transfer in a photocatalytic membrane will be useful in suppressing undesired side reactions (e.g., re-oxidation of a reduced product) and facilitating product separation.
578. Extending Infrared Emission via Energy Transfer in a CsPbI3–Cyanine Dye Hybrid Jishnudas Chakkamalayath, Lauren E. Martin, and Prashant V. Kamat J. Phys. Chem. Lett. 2024, 15, 2, 401-407.
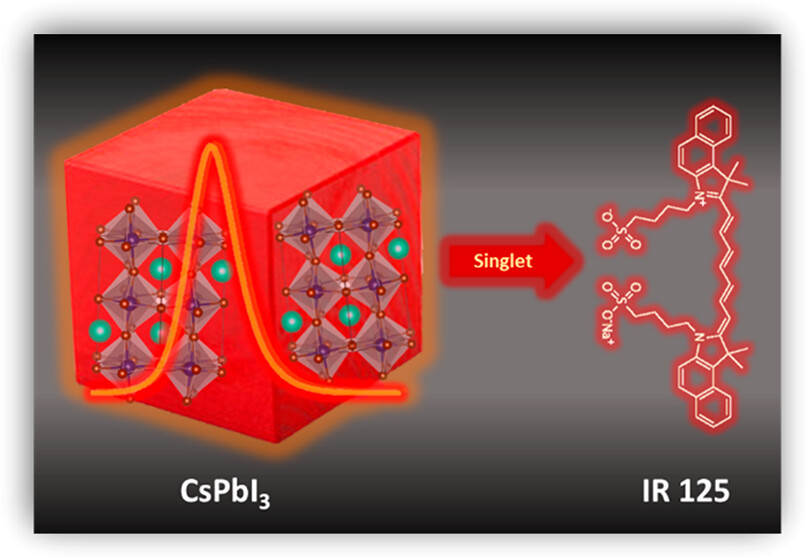 Directing energy flow in light harvesting assemblies of nanocrystal–chromophore hybrid systems requires a better understanding of factors that dictate excited-state processes. In this study, we explore excited-state interactions within the CsPbI3–cyanine dye (IR125) hybrid assembly through a comprehensive set of steady-state and time-resolved absorption and photoluminescence (PL) experiments. Our photoluminescence investigations reveal the quenching of CsPbI3 emission alongside the simultaneous enhancement of IR125 fluorescence, providing evidence for a singlet energy transfer. The evaluation of both photoluminescence (PL) quenching and PL decay measurements yield ∼94% energy transfer efficiency for the CsPbI3–IR125 hybrid assembly. Transient absorption spectroscopy further unveils that this singlet energy transfer process operates on an ultrafast time scale, occurring within 400 ps with a rate constant of energy transfer of 1.4 × 1010 s-1. Our findings highlight the potential of the CsPbI3–IR125 hybrid assembly to extend the emission of halide perovskites into the infrared region, paving the way for light energy harvesting and display applications.
Directing energy flow in light harvesting assemblies of nanocrystal–chromophore hybrid systems requires a better understanding of factors that dictate excited-state processes. In this study, we explore excited-state interactions within the CsPbI3–cyanine dye (IR125) hybrid assembly through a comprehensive set of steady-state and time-resolved absorption and photoluminescence (PL) experiments. Our photoluminescence investigations reveal the quenching of CsPbI3 emission alongside the simultaneous enhancement of IR125 fluorescence, providing evidence for a singlet energy transfer. The evaluation of both photoluminescence (PL) quenching and PL decay measurements yield ∼94% energy transfer efficiency for the CsPbI3–IR125 hybrid assembly. Transient absorption spectroscopy further unveils that this singlet energy transfer process operates on an ultrafast time scale, occurring within 400 ps with a rate constant of energy transfer of 1.4 × 1010 s-1. Our findings highlight the potential of the CsPbI3–IR125 hybrid assembly to extend the emission of halide perovskites into the infrared region, paving the way for light energy harvesting and display applications.
577. AgInS2-Embedded Photocatalytic Membrane: Insights into the Excited State and Electron Transfer Dynamics Anthony Kipkorir, Gavin Ealey, Yiseul Yu, and Prashant V. Kamat Langmuir 2024, 40, 2, 1373-1380.
 Photocatalytic reactions at semiconductor nanocrystal surfaces are useful for synthesizing value-added chemicals using sunlight. Semiconductor nanocrystals dispersed in a rigid framework, such as polymer film, can mitigate issues such as aggregation, product separation, and other challenges that are usually encountered in suspensions or slurries. Using a cation exchange technique, we successfully embedded AgInS2 nanoparticles into a Nafion matrix, termed AgInS2–Nafion. This was achieved through a galvanic exchange between In and Ag in In2S3 present within the Nafion film, enabling an adjustable Ag:In ratio for optimized photophysical properties. As in the case of colloidal suspension, the AgInS2 particles embedded in Nafion exhibit a long absorption tail, a broad emission band with a large Stokes shift, and emission lifetimes extending into the microseconds that are characteristic of donor–acceptor pairs, DAP. Remediation of surface states with the treatment of 3-mercaptopropionic acid resulted in significant enhancement in the emission yield. Charge carrier generation through bandgap excitation as well as activation of DAP states which reside within the bandgap is probed through transient absorption spectroscopy. The photocatalytic activity of AgInS2–Nafion was probed by using thionine as an electron acceptor. The electron transfer rate constant from excited AgInS2 to thionine as observed from transient absorption spectroscopy was determined to be ∼6.3 × 1010 s-1. The design of a photoactive membrane offers new ways to carry out photocatalytic processes with greater selectivity.
Photocatalytic reactions at semiconductor nanocrystal surfaces are useful for synthesizing value-added chemicals using sunlight. Semiconductor nanocrystals dispersed in a rigid framework, such as polymer film, can mitigate issues such as aggregation, product separation, and other challenges that are usually encountered in suspensions or slurries. Using a cation exchange technique, we successfully embedded AgInS2 nanoparticles into a Nafion matrix, termed AgInS2–Nafion. This was achieved through a galvanic exchange between In and Ag in In2S3 present within the Nafion film, enabling an adjustable Ag:In ratio for optimized photophysical properties. As in the case of colloidal suspension, the AgInS2 particles embedded in Nafion exhibit a long absorption tail, a broad emission band with a large Stokes shift, and emission lifetimes extending into the microseconds that are characteristic of donor–acceptor pairs, DAP. Remediation of surface states with the treatment of 3-mercaptopropionic acid resulted in significant enhancement in the emission yield. Charge carrier generation through bandgap excitation as well as activation of DAP states which reside within the bandgap is probed through transient absorption spectroscopy. The photocatalytic activity of AgInS2–Nafion was probed by using thionine as an electron acceptor. The electron transfer rate constant from excited AgInS2 to thionine as observed from transient absorption spectroscopy was determined to be ∼6.3 × 1010 s-1. The design of a photoactive membrane offers new ways to carry out photocatalytic processes with greater selectivity.
576. How Cation Migration across a 2D/3D Interface Dictates Perovskite Solar Cell Efficiency Gábor Szabó and Prashant V. Kamat ACS Energy Lett. 2024, 9, 1, 193-200.
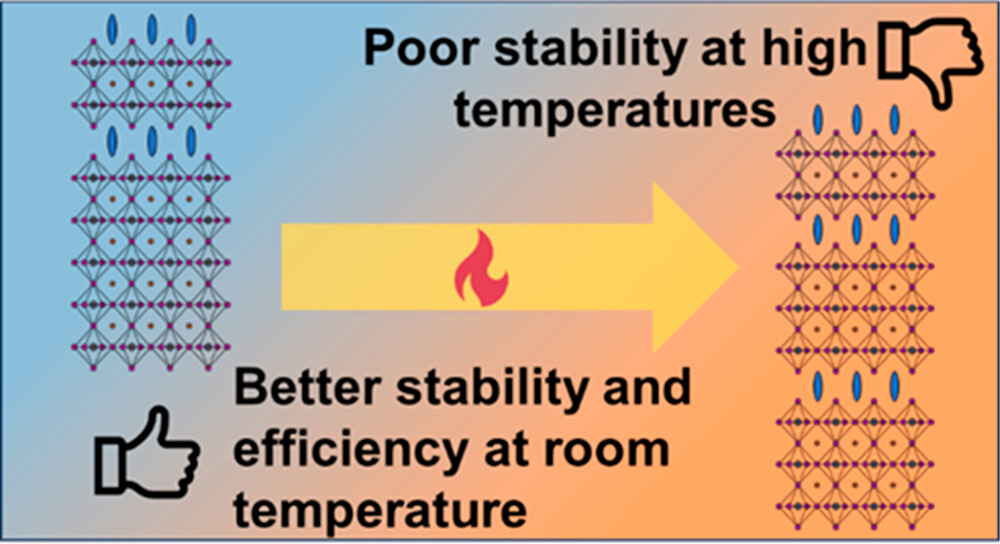 Perovskite solar cells with 2D/3D architecture are claimed to exhibit better stability compared to pristine 3D films at room temperature. However, under illumination and/or heat, cation migration causes the exchange of the bulky spacer cations (2D phase) with the smaller A-site cations (3D phase), creating a gradient heterostructure at the 2D/3D interface. We have evaluated the performance of BA2MAPb2I7/MAPbI3 (2D/3D) and MAPbI3 (3D) solar cells at different temperatures, while simultaneously probing the absorption changes of the 2D/3D perovskite layer and the photovoltaic performance of solar cell devices. The 2D/3D solar cells were more stable at room temperature but exhibited deterioration of photovoltaic performance at high temperatures. By employing in situ measurements of operating solar cells to track both the photoconversion efficiency and absorption changes at different temperatures, we show that the cation exchange at the 2D/3D interface contributes to the efficiency losses.
Perovskite solar cells with 2D/3D architecture are claimed to exhibit better stability compared to pristine 3D films at room temperature. However, under illumination and/or heat, cation migration causes the exchange of the bulky spacer cations (2D phase) with the smaller A-site cations (3D phase), creating a gradient heterostructure at the 2D/3D interface. We have evaluated the performance of BA2MAPb2I7/MAPbI3 (2D/3D) and MAPbI3 (3D) solar cells at different temperatures, while simultaneously probing the absorption changes of the 2D/3D perovskite layer and the photovoltaic performance of solar cell devices. The 2D/3D solar cells were more stable at room temperature but exhibited deterioration of photovoltaic performance at high temperatures. By employing in situ measurements of operating solar cells to track both the photoconversion efficiency and absorption changes at different temperatures, we show that the cation exchange at the 2D/3D interface contributes to the efficiency losses.
2023
575. Cation Migration in Physically Paired 2D and 3D Lead Halide Perovskite Films Preethi S. Mathew and Prashant V. Kamat Adv. Optical Mater. 2023, 2300957.
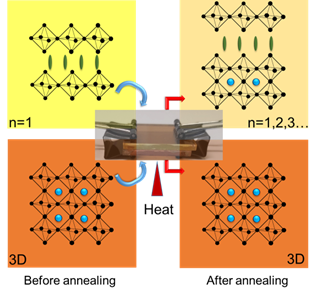 2D/3D interfaces provide long-term stability for the operation of metal halide perovskite solar cells. However, the cation migration under heat and light can disturb the interface and create a graded cation interface. This study has now probed the cation migration by physically pairing X2PbI4 (X = butylammonium BA, oleylammonium OA, or phenethylammonium PEA) 2D film and (CH3NH3, MA)PbI3 3D film at different temperatures and recording changes in the absorption and emission spectra. The migration of the methyl ammonium cation toward the 2D film slowly transforms the n = 1 layered phase into n = 2 and 3 layered phases. The 3D film, on the other hand, exhibits relatively small changes, as the inclusion of spacer cation has little effect on its phase. The apparent activation energy determined from the temperature-dependent cation migration kinetics (Ea = 29– 50 kJ mol-1) indicates alkyl ammonium cations such as butyl ammonium migrate more readily into the 3D layer than aromatic cations such as PEA. The ease of migration of spacer cations between physically paired films suggests that the varied composition at the interface should be considered while evaluating the effectiveness of the 2D/3D design strategy for improving the performance of perovskite solar cells.
2D/3D interfaces provide long-term stability for the operation of metal halide perovskite solar cells. However, the cation migration under heat and light can disturb the interface and create a graded cation interface. This study has now probed the cation migration by physically pairing X2PbI4 (X = butylammonium BA, oleylammonium OA, or phenethylammonium PEA) 2D film and (CH3NH3, MA)PbI3 3D film at different temperatures and recording changes in the absorption and emission spectra. The migration of the methyl ammonium cation toward the 2D film slowly transforms the n = 1 layered phase into n = 2 and 3 layered phases. The 3D film, on the other hand, exhibits relatively small changes, as the inclusion of spacer cation has little effect on its phase. The apparent activation energy determined from the temperature-dependent cation migration kinetics (Ea = 29– 50 kJ mol-1) indicates alkyl ammonium cations such as butyl ammonium migrate more readily into the 3D layer than aromatic cations such as PEA. The ease of migration of spacer cations between physically paired films suggests that the varied composition at the interface should be considered while evaluating the effectiveness of the 2D/3D design strategy for improving the performance of perovskite solar cells.
574. Thermodynamic Band Gap Model for Photoinduced Phase Segregation in Mixed-Halide Perovskites Anthony Ruth, Halyna Okrepka, Prashant V. Kamat, and Masaru Kuno J. Phys. Chem. C. 2023, 127, 37, 18547-18559
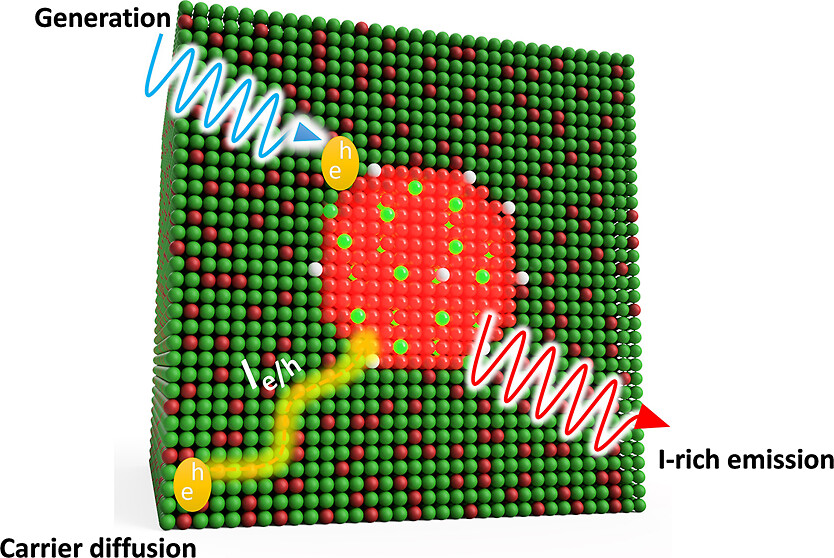 Provided is a comprehensive description of a band gap thermodynamic model, which predicts and explains key features of photosegregation in lead-based, mixed-halide perovskites. The model gives a prescription for illustrating halide migration driven by photocarrier energies. Where possible, model predictions are compared with experimental results. Free energy derivations are provided for three assumptions: (1) halide mixing in the dark, (2) a fixed number of photogenerated carriers funneling to and localizing in low band gap inclusions of the alloy, and (3) the statistical occupancy of said inclusions from a bath of thermalized photocarriers in the parent material. Model predictions include excitation intensity (Iexc)-dependent terminal halide stoichiometries (xterminal), temperature-independent xterminal, excitation intensity thresholds (Iexc,threshold) below which photosegregation is suppressed, and reduced segregation in nanocrystals as compared to thin films. The model also offers insight into kinetically manipulating photosegregation rates via control of underlying mediators and rationalizes asymmetries in forward and reverse photosegregation rate constants/activation energies. What emerges is a cohesive framework for understanding ubiquitous photosegregation in mixed-halide perovskites and a rational basis by which to manage the phenomenon.
Provided is a comprehensive description of a band gap thermodynamic model, which predicts and explains key features of photosegregation in lead-based, mixed-halide perovskites. The model gives a prescription for illustrating halide migration driven by photocarrier energies. Where possible, model predictions are compared with experimental results. Free energy derivations are provided for three assumptions: (1) halide mixing in the dark, (2) a fixed number of photogenerated carriers funneling to and localizing in low band gap inclusions of the alloy, and (3) the statistical occupancy of said inclusions from a bath of thermalized photocarriers in the parent material. Model predictions include excitation intensity (Iexc)-dependent terminal halide stoichiometries (xterminal), temperature-independent xterminal, excitation intensity thresholds (Iexc,threshold) below which photosegregation is suppressed, and reduced segregation in nanocrystals as compared to thin films. The model also offers insight into kinetically manipulating photosegregation rates via control of underlying mediators and rationalizes asymmetries in forward and reverse photosegregation rate constants/activation energies. What emerges is a cohesive framework for understanding ubiquitous photosegregation in mixed-halide perovskites and a rational basis by which to manage the phenomenon.
573. Directing Singlet Excited Energy Flow in Rubrene-Perylene Dye (DBP) Films Jishnudas Chakkamalayath and Prashant V. Kamat J. Phys. Chem. C. 2023, 127, 33, 16312-16318
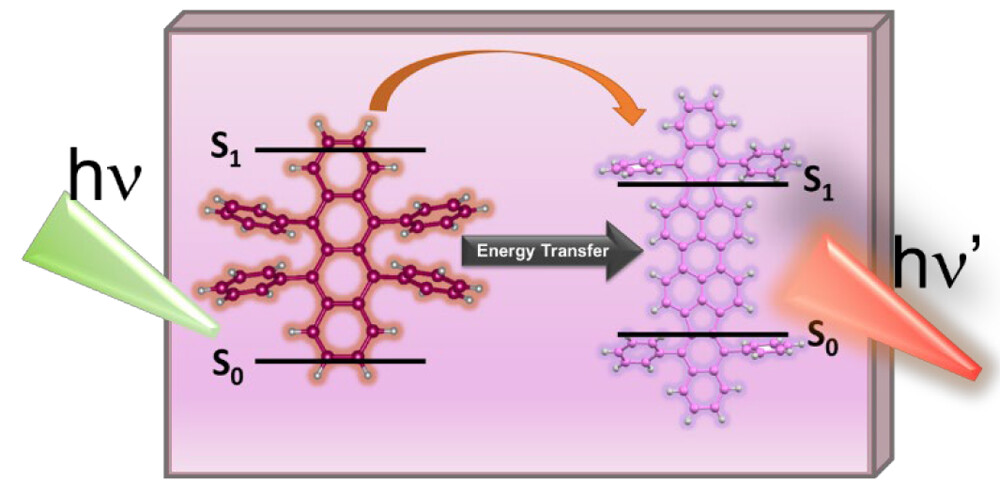 Energy transfer between a sensitizer and a triplet annihilator-emitter system is a key process that dictates the design of a light energy upconversion system. Using a combination of steady-state and time-resolved emission experiments, we have now elucidated excited-state interactions between the rubrene dye and a perylene derivative (DBP), which are commonly employed in upconversion molecular assemblies. Quenching of rubrene emission with a concomitant increase in DBP emission in rubrene-DBP films confirms singlet-energy transfer with a singlet energy transfer efficiency as high as 94%. Under low-intensity (steady-state) excitation, the singlet excited state of rubrene acts as a primary donor in the Förster resonance energy transfer process. The rate constant of energy transfer as determined from emission lifetime measurements of rubrene-DBP films was 1.4 × 109 s-1. The factors that control the singlet energy transfer between excited rubrene and DBP and its role in designing light energy harvesting assemblies are discussed.
Energy transfer between a sensitizer and a triplet annihilator-emitter system is a key process that dictates the design of a light energy upconversion system. Using a combination of steady-state and time-resolved emission experiments, we have now elucidated excited-state interactions between the rubrene dye and a perylene derivative (DBP), which are commonly employed in upconversion molecular assemblies. Quenching of rubrene emission with a concomitant increase in DBP emission in rubrene-DBP films confirms singlet-energy transfer with a singlet energy transfer efficiency as high as 94%. Under low-intensity (steady-state) excitation, the singlet excited state of rubrene acts as a primary donor in the Förster resonance energy transfer process. The rate constant of energy transfer as determined from emission lifetime measurements of rubrene-DBP films was 1.4 × 109 s-1. The factors that control the singlet energy transfer between excited rubrene and DBP and its role in designing light energy harvesting assemblies are discussed.
572. Reversible Phase Transformation of Colloidal 2D Lead Halide Perovskite Platelets under Photoirradiation Nathaniel Hiott, Jishnudas Chakkamalayath and Prashant V. Kamat ACS Materials Lett. 2023, 5, 10, 2614-2620
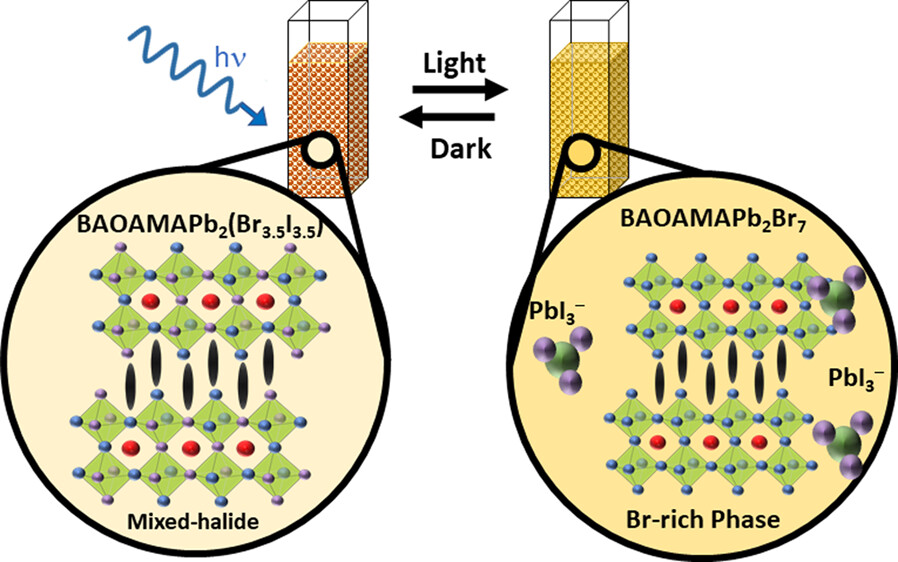 The photostability of lead halide perovskites is an important criterion for their utilization in light harvesting applications. Colloidal two-dimensional mixed halide perovskites offer a convenient approach to probe photoinduced transformations using spectroscopy tools. We employed suspensions of colloidal 2D methylammonium lead halide perovskite platelets with butylammonium and octylammonium as organic spacer cations. Upon irradiation of the mixed halide perovskite (BAOAMAPb2(Br3.5I3.5)) colloidal platelet suspension, we observe a phase transformation, as seen from the formation of bromide rich domains (BAOAMAPb2Br7) and an iodide complex (PbI3–). Unlike in the case of 3D mixed halide films, we did not observe the formation of iodide rich domains as part of the photoinduced phase segregation process. Instead, we observed the dissociation of the BAOAMAPb2I7 to form an iodide complex, PbI3–. Interestingly, this photoinduced phase transformation was reversible, as the products which were confined in the colloidal core restored the original mixed halide phase upon storage in dark. The spectroscopic measurements that characterize excited states of different colloidal methylammonium lead halide perovskites and kinetics of photoinduced transformation and their dark recovery are presented. These new phototransformations of colloidal perovskite platelets provide new insights into the processes that dictate their overall photostability.
The photostability of lead halide perovskites is an important criterion for their utilization in light harvesting applications. Colloidal two-dimensional mixed halide perovskites offer a convenient approach to probe photoinduced transformations using spectroscopy tools. We employed suspensions of colloidal 2D methylammonium lead halide perovskite platelets with butylammonium and octylammonium as organic spacer cations. Upon irradiation of the mixed halide perovskite (BAOAMAPb2(Br3.5I3.5)) colloidal platelet suspension, we observe a phase transformation, as seen from the formation of bromide rich domains (BAOAMAPb2Br7) and an iodide complex (PbI3–). Unlike in the case of 3D mixed halide films, we did not observe the formation of iodide rich domains as part of the photoinduced phase segregation process. Instead, we observed the dissociation of the BAOAMAPb2I7 to form an iodide complex, PbI3–. Interestingly, this photoinduced phase transformation was reversible, as the products which were confined in the colloidal core restored the original mixed halide phase upon storage in dark. The spectroscopic measurements that characterize excited states of different colloidal methylammonium lead halide perovskites and kinetics of photoinduced transformation and their dark recovery are presented. These new phototransformations of colloidal perovskite platelets provide new insights into the processes that dictate their overall photostability.
571. Trap or Triplet? Excited–State Interactions in 2D Perovskite Colloids with Chromophoric Cations Jeffrey T. DuBose, Andrew Christy, Jishnudas Chakkamalayath and Prashant V. Kamat ACS Nano 2023, 17, 19, 19052-19062
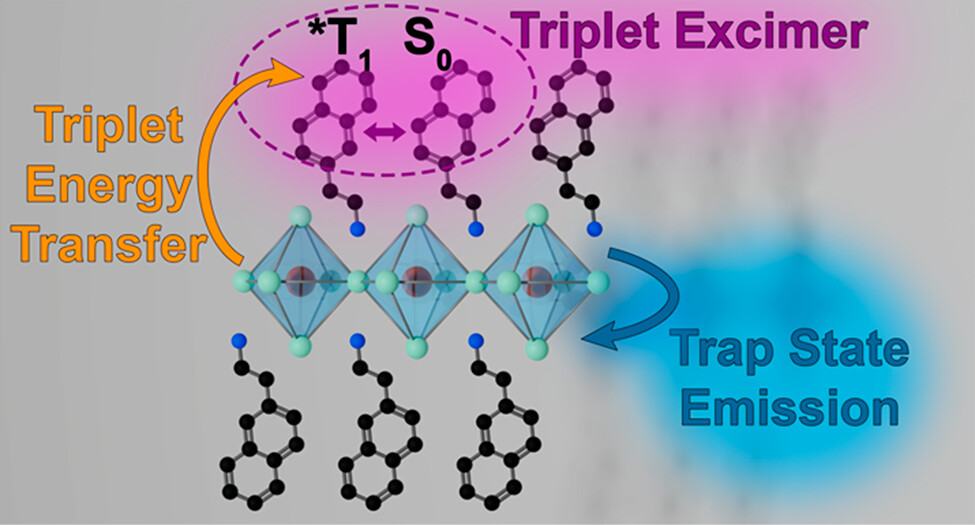 Movement of energy within light-harvesting assemblies is typically carried out with separately synthesized donor and acceptor species, which are then brought together to induce an interaction. Recently, two-dimensional (2D) lead halide perovskites have gained interest for their ability to accommodate and assemble chromophoric molecules within their lattice, creating hybrid organic–inorganic compositions. Using a combination of steady-state and time-resolved absorption and emission spectroscopy, we have now succeeded in establishing the competition between energy transfer and charge trapping in 2D halide perovskite colloids containing naphthalene-derived cations (i.e., NEA2PbX4, where NEA = naphthylethylamine). The presence of room-temperature triplet emission from the naphthalene moiety depends on the ratio of bromide to iodide in the lead halide sublattice (i.e., x in NEA2Pb(Br1–xIx)4), with only bromide-rich compositions showing sensitized emission. Photoluminescence lifetime measurements of the sensitized naphthalene reveal the formation of the naphthalene triplet excimer at room temperature. From transient absorption measurements, we find the rate constant of triplet energy transfer (kEnT) to be on the order of ∼109 s–1. At low temperatures (77 K) a new broad emission feature arising from trap states is observed in all samples ranging from pure bromide to pure iodide composition. These results reveal the interplay between sensitized triplet energy transfer and charge trapping in 2D lead halide perovskites, highlighting the need to carefully parse contributions from competing de-excitation pathways for optoelectronic applications.
Movement of energy within light-harvesting assemblies is typically carried out with separately synthesized donor and acceptor species, which are then brought together to induce an interaction. Recently, two-dimensional (2D) lead halide perovskites have gained interest for their ability to accommodate and assemble chromophoric molecules within their lattice, creating hybrid organic–inorganic compositions. Using a combination of steady-state and time-resolved absorption and emission spectroscopy, we have now succeeded in establishing the competition between energy transfer and charge trapping in 2D halide perovskite colloids containing naphthalene-derived cations (i.e., NEA2PbX4, where NEA = naphthylethylamine). The presence of room-temperature triplet emission from the naphthalene moiety depends on the ratio of bromide to iodide in the lead halide sublattice (i.e., x in NEA2Pb(Br1–xIx)4), with only bromide-rich compositions showing sensitized emission. Photoluminescence lifetime measurements of the sensitized naphthalene reveal the formation of the naphthalene triplet excimer at room temperature. From transient absorption measurements, we find the rate constant of triplet energy transfer (kEnT) to be on the order of ∼109 s–1. At low temperatures (77 K) a new broad emission feature arising from trap states is observed in all samples ranging from pure bromide to pure iodide composition. These results reveal the interplay between sensitized triplet energy transfer and charge trapping in 2D lead halide perovskites, highlighting the need to carefully parse contributions from competing de-excitation pathways for optoelectronic applications.
570. Photoinduced electron transfer across the polymer-capped CsPbBr3 interface in a polar medium Anthony Kipkorir, Xiuyu Jin, Haifeng Gao and Prashant V. Kamat J. Chem. Phys. 2023, 158, 14, 144702
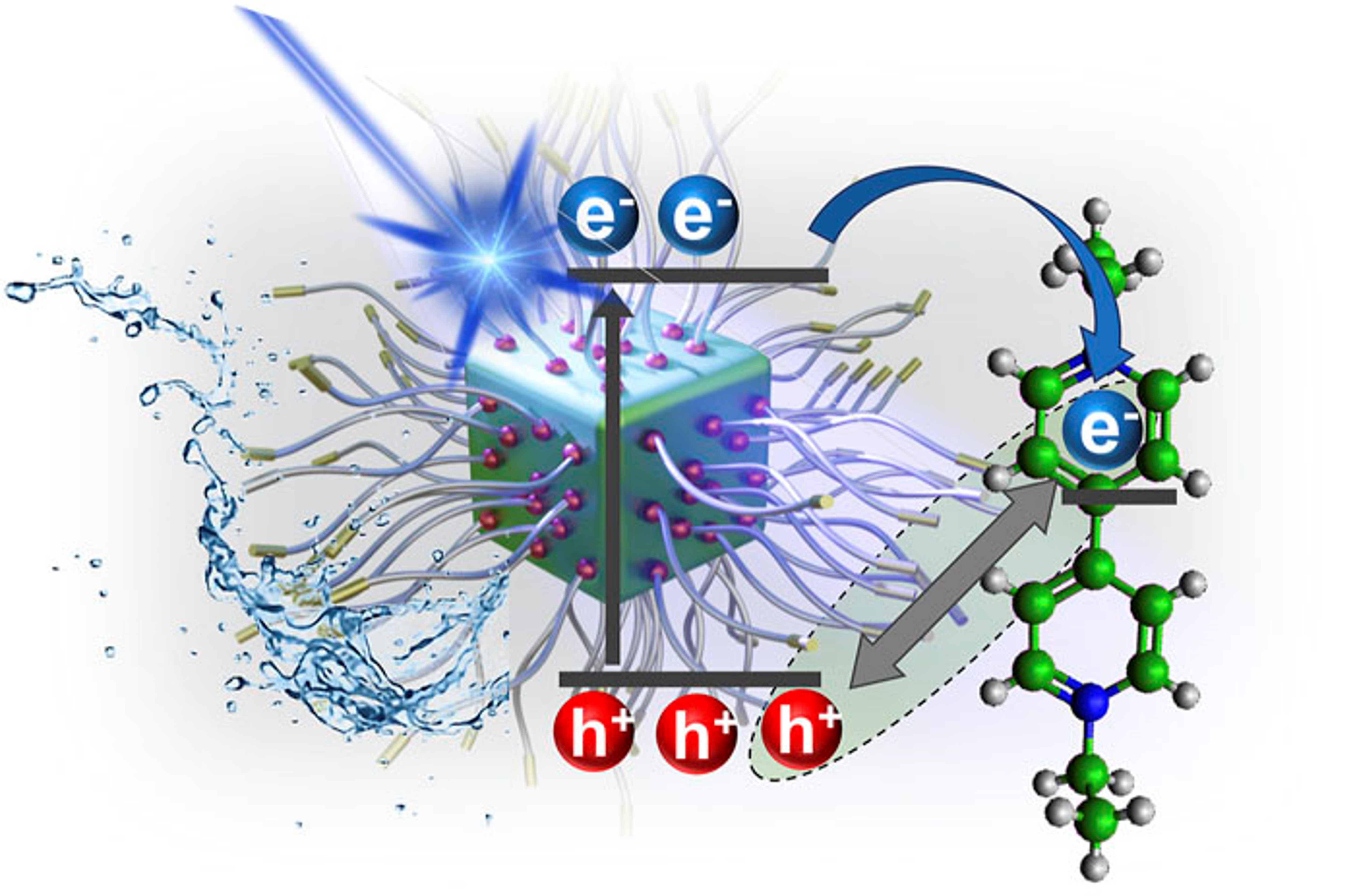 In-situ polymer capping of cesium lead bromide (CsPbBr3) nanocrystals with polymethyl acrylate is an effective approach to improve the colloidal stability in the polar medium and thus extends their use in photocatalysis. The photoinduced electron transfer properties of polymethyl acrylate (PMA)-capped CsPbBr3 nanocrystals have been probed using surface-bound viologen molecules with different alkyl chains as electron acceptors. The apparent association constant (Kapp) obtained for the binding of viologen molecules with PMA-capped CsPbBr3 was 2.3 × 107 M-1, which is an order of magnitude greater than that obtained with oleic acid/oleylamine-capped CsPbBr3. Although the length of the alkyl chain of the viologen molecule did not show any impact on the electron transfer rate constant, it influenced the charge separation efficiency and net electron transfer quantum yield. Viologen moieties with a shorter alkyl chain length exhibited a charge separation efficiency of 72% compared with 50% for the longer chain alkyl chain length viologens. Implications of polymer-capped CsPbBr3 perovskite nanocrystals for carrying out photocatalytic reduction in the polar medium are discussed.
In-situ polymer capping of cesium lead bromide (CsPbBr3) nanocrystals with polymethyl acrylate is an effective approach to improve the colloidal stability in the polar medium and thus extends their use in photocatalysis. The photoinduced electron transfer properties of polymethyl acrylate (PMA)-capped CsPbBr3 nanocrystals have been probed using surface-bound viologen molecules with different alkyl chains as electron acceptors. The apparent association constant (Kapp) obtained for the binding of viologen molecules with PMA-capped CsPbBr3 was 2.3 × 107 M-1, which is an order of magnitude greater than that obtained with oleic acid/oleylamine-capped CsPbBr3. Although the length of the alkyl chain of the viologen molecule did not show any impact on the electron transfer rate constant, it influenced the charge separation efficiency and net electron transfer quantum yield. Viologen moieties with a shorter alkyl chain length exhibited a charge separation efficiency of 72% compared with 50% for the longer chain alkyl chain length viologens. Implications of polymer-capped CsPbBr3 perovskite nanocrystals for carrying out photocatalytic reduction in the polar medium are discussed.
569. How Pendant Groups Dictate Energy and Electron Transfer in Perovskite–Rhodamine Light Harvesting Assemblies Jeffrey T. DuBose and Prashant V. Kamat J. Am. Chem. Soc. 2023, 145, 8, 4601-4612
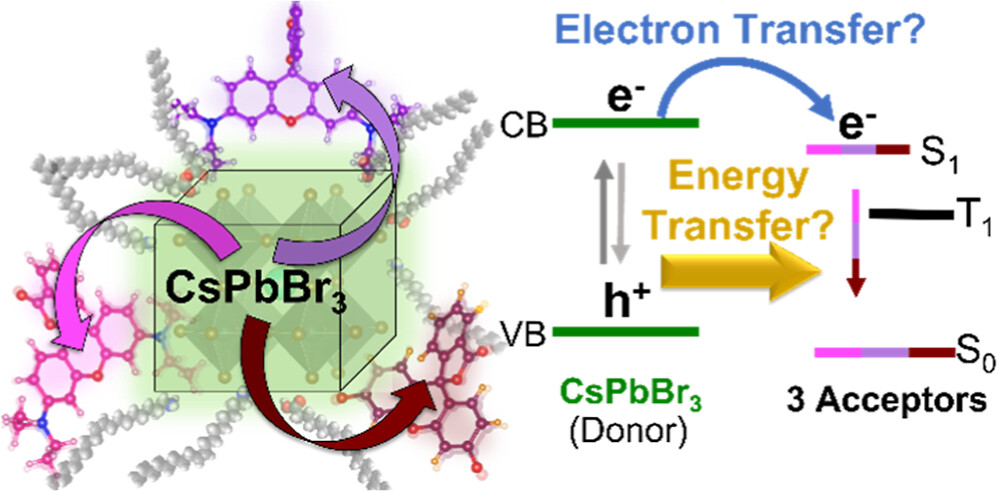 Energy and electron transfer processes allow for efficient manipulation of excited states within light harvesting assemblies for photocatalytic and optoelectronic applications. We have now successfully probed the influence of acceptor pendant group functionalization on the energy and electron transfer between CsPbBr3 perovskite nanocrystals and three rhodamine-based acceptor molecules. The three acceptors─rhodamine B (RhB), rhodamine isothiocyanate (RhB-NCS), and rose Bengal (RoseB)─contain an increasing degree of pendant group functionalization that affects their native excited state properties. When interacting with CsPbBr3 as an energy donor, photoluminescence excitation spectroscopy reveals that singlet energy transfer occurs with all three acceptors. However, the acceptor functionalization directly influences several key parameters that dictate the excited state interactions. For example, RoseB binds to the nanocrystal surface with an apparent association constant (Kapp = 9.4 × 106 M-1) 200 times greater than RhB (Kapp = 0.05 × 106 M-1), thus influencing the rate of energy transfer. Femtosecond transient absorption reveals the observed rate constant of singlet energy transfer (kEnT) is an order-of-magnitude greater for RoseB (kEnT = 1 × 1011 s-1) than for RhB and RhB-NCS. In addition to energy transfer, each acceptor had a subpopulation of molecules (∼30%) that underwent electron transfer as a competing pathway. Thus, the structural influence of acceptor moieties must be considered for both excited state energy and electron transfer in nanocrystal-molecular hybrids. The competition between electron and energy transfer further highlights the complexity of excited state interactions in nanocrystal-molecular complexes and the need for careful spectroscopic analysis to elucidate competitive pathways.
Energy and electron transfer processes allow for efficient manipulation of excited states within light harvesting assemblies for photocatalytic and optoelectronic applications. We have now successfully probed the influence of acceptor pendant group functionalization on the energy and electron transfer between CsPbBr3 perovskite nanocrystals and three rhodamine-based acceptor molecules. The three acceptors─rhodamine B (RhB), rhodamine isothiocyanate (RhB-NCS), and rose Bengal (RoseB)─contain an increasing degree of pendant group functionalization that affects their native excited state properties. When interacting with CsPbBr3 as an energy donor, photoluminescence excitation spectroscopy reveals that singlet energy transfer occurs with all three acceptors. However, the acceptor functionalization directly influences several key parameters that dictate the excited state interactions. For example, RoseB binds to the nanocrystal surface with an apparent association constant (Kapp = 9.4 × 106 M-1) 200 times greater than RhB (Kapp = 0.05 × 106 M-1), thus influencing the rate of energy transfer. Femtosecond transient absorption reveals the observed rate constant of singlet energy transfer (kEnT) is an order-of-magnitude greater for RoseB (kEnT = 1 × 1011 s-1) than for RhB and RhB-NCS. In addition to energy transfer, each acceptor had a subpopulation of molecules (∼30%) that underwent electron transfer as a competing pathway. Thus, the structural influence of acceptor moieties must be considered for both excited state energy and electron transfer in nanocrystal-molecular hybrids. The competition between electron and energy transfer further highlights the complexity of excited state interactions in nanocrystal-molecular complexes and the need for careful spectroscopic analysis to elucidate competitive pathways.
568. How Stable Is the 2D/3D Interface of Metal Halide Perovskite under Light and Heat? Jishnudas Chakkamalayath, Nathaniel Hiott, and Prashant V. Kamat ACS Energy Lett. 2023, 8, 1, 169-171
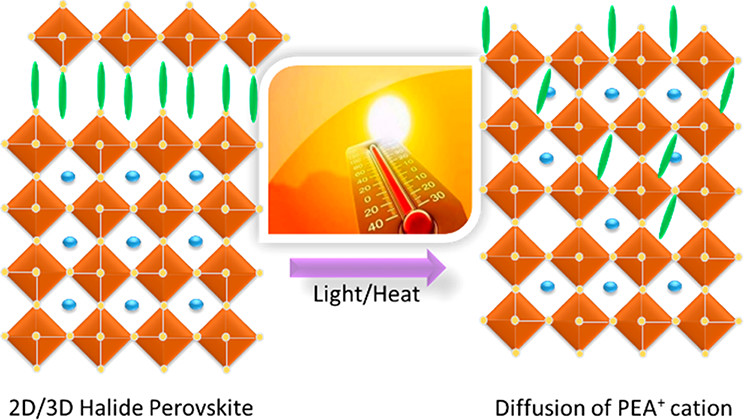 A (PEA)2PbI4 (2D) layer deposited on a MAPbI3 (3D) film undergoes a transformation upon photoirradiation and thermal treatment. The decrease in absorption and emission of the 2D layer during light and heat exposure, which shows migration of the PEA+ cation within the 3D film, points out the dynamic nature of the 2D/3D interface and intermixing of the two phases.
A (PEA)2PbI4 (2D) layer deposited on a MAPbI3 (3D) film undergoes a transformation upon photoirradiation and thermal treatment. The decrease in absorption and emission of the 2D layer during light and heat exposure, which shows migration of the PEA+ cation within the 3D film, points out the dynamic nature of the 2D/3D interface and intermixing of the two phases.
567. Excited State and Transient Chemistry of a Perylene Derivative (DBP). An Untold Story Jishnudas Chakkamalayath, Gábor Szabó, Jeffrey T. DuBose, and Prashant V. Kamat J. Phys. Chem. A 2023, 127, 1, 99-106
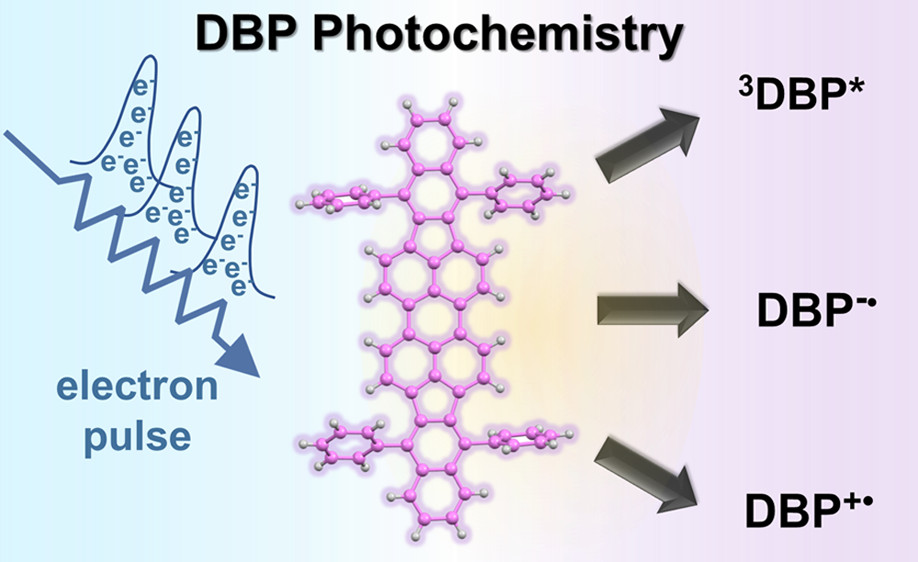 Transient chemistry of sensitizing dyes is important to obtain insights into the photochemical conversion processes of light harvesting assemblies. We have now employed transient absorption spectroscopy (pulsed laser and pulse radiolysis) to characterize the excited state and radical intermediates of a perylene derivative, (5,10,15,20-Tetraphenylbisbenz[5,6]indeno[1,2,3-cd:1′,2′,3′-lm]perylene (DBP). The distinguishable transient absorption features for the singlet and triplet excited states and radical anion and radical cation provide spectral fingerprints to identify the reaction intermediates in photochemical energy and electron transfer processes of composite systems involving DBP. For example, identifying these transients in the energy transfer processes of the rubrene–DBP system would aid in establishing their role as annihilator-emitter for triplet–triplet annihilation up-conversion (TTA-UC). The transient characterization thus serves as an important mechanistic fingerprint for elucidating mechanistic details of systems employing DBP in optoelectronic applications.
Transient chemistry of sensitizing dyes is important to obtain insights into the photochemical conversion processes of light harvesting assemblies. We have now employed transient absorption spectroscopy (pulsed laser and pulse radiolysis) to characterize the excited state and radical intermediates of a perylene derivative, (5,10,15,20-Tetraphenylbisbenz[5,6]indeno[1,2,3-cd:1′,2′,3′-lm]perylene (DBP). The distinguishable transient absorption features for the singlet and triplet excited states and radical anion and radical cation provide spectral fingerprints to identify the reaction intermediates in photochemical energy and electron transfer processes of composite systems involving DBP. For example, identifying these transients in the energy transfer processes of the rubrene–DBP system would aid in establishing their role as annihilator-emitter for triplet–triplet annihilation up-conversion (TTA-UC). The transient characterization thus serves as an important mechanistic fingerprint for elucidating mechanistic details of systems employing DBP in optoelectronic applications.
2022
566. Enhanced Charge Carrier Separation in WO3/BiVO4 Photoanodes Achieved via Light Absorption in the BiVO4 Layer Ivan Grigioni, Annalisa Polo, Maria Vittoria Dozzi, Kevin G. Stamplecoskie, Danilo H. Jara, Prashant V. Kamat, and Elena Selli ACS Appl. Energy Mater. 2022, 5, 11, 13142-13148
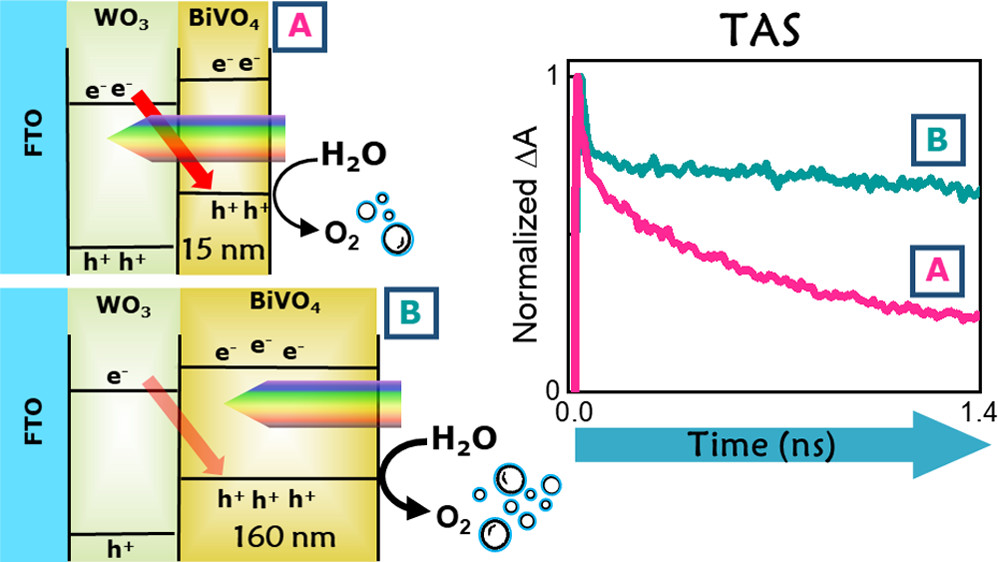 Photoelectrochemical (PEC) water splitting converts solar light and water into oxygen and energy-rich hydrogen. WO3/BiVO4 heterojunction photoanodes perform much better than the separate oxide components, though internal charge recombination undermines their PEC performance when both oxides absorb light. Here we exploit the BiVO4 layer to sensitize WO4 to visible light and shield it from direct photoexcitation to overcome this efficiency loss. PEC experiments and ultrafast transient absorption spectroscopy performed by frontside (through BiVO4) or backside (through WO3) irradiating photoanodes with different BiVO4 layer thickness demonstrate that irradiation through BiVO4 is beneficial for charge separation. Optimized electrodes irradiated through BiVO4 show 40% higher photocurrent density compared to backside irradiation.
Photoelectrochemical (PEC) water splitting converts solar light and water into oxygen and energy-rich hydrogen. WO3/BiVO4 heterojunction photoanodes perform much better than the separate oxide components, though internal charge recombination undermines their PEC performance when both oxides absorb light. Here we exploit the BiVO4 layer to sensitize WO4 to visible light and shield it from direct photoexcitation to overcome this efficiency loss. PEC experiments and ultrafast transient absorption spectroscopy performed by frontside (through BiVO4) or backside (through WO3) irradiating photoanodes with different BiVO4 layer thickness demonstrate that irradiation through BiVO4 is beneficial for charge separation. Optimized electrodes irradiated through BiVO4 show 40% higher photocurrent density compared to backside irradiation.
565. Phase Segregation and Sequential Expulsion of Iodide and Bromide in Photoirradiated Ruddlesden–Popper 2D Perovskite Films Preethi Susan Mathew, Gábor Szabó, Masaru Kuno, and Prashant V. Kamat ACS Energy Lett. 2022, 7, 11, 3982–3988
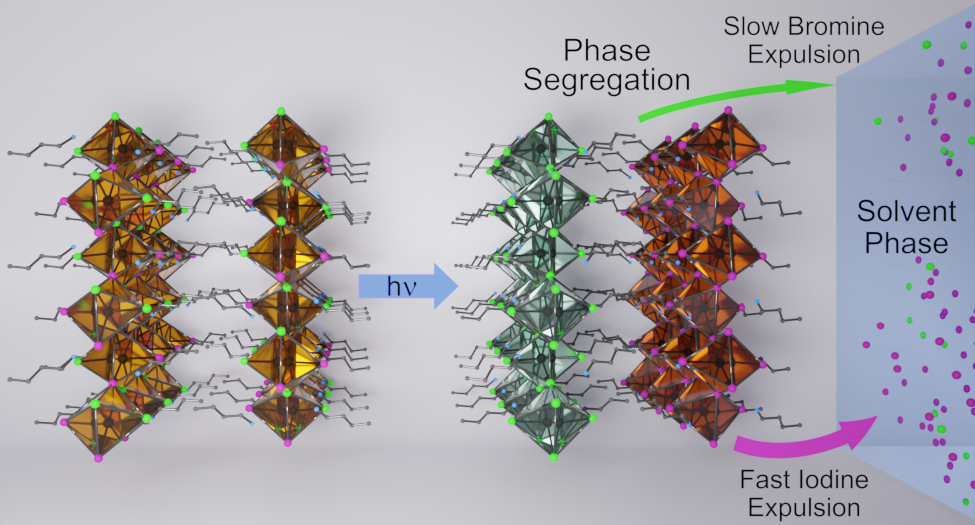 Two-dimensional (2D) Ruddlesden–Popper mixed-halide perovskite films, BA2PbBr2I2, undergo phase segregation when excited with visible light to generate bromide- and iodide-rich regions, as marked by absorption and emission changes. Upon stopping illumination, the process reverses, allowing original film compositions to be restored. However, if films are in contact with dichloromethane, light irradiation causes the sequential expulsion of iodide and bromide and introduces irreversible changes to the 2D films. The sequential disappearance of I- and Br- from pristine films (BA2PbBr4 and BA2PbI4) under photoirradiation, as observed from variances in expulsion rates, reflects differences in halide ion mobilities in these films. The photoinstability of 2D films raises questions about their use in stabilizing bulk, three-dimensional (3D) perovskite solar cells through 3D/2D interfaces.
Two-dimensional (2D) Ruddlesden–Popper mixed-halide perovskite films, BA2PbBr2I2, undergo phase segregation when excited with visible light to generate bromide- and iodide-rich regions, as marked by absorption and emission changes. Upon stopping illumination, the process reverses, allowing original film compositions to be restored. However, if films are in contact with dichloromethane, light irradiation causes the sequential expulsion of iodide and bromide and introduces irreversible changes to the 2D films. The sequential disappearance of I- and Br- from pristine films (BA2PbBr4 and BA2PbI4) under photoirradiation, as observed from variances in expulsion rates, reflects differences in halide ion mobilities in these films. The photoinstability of 2D films raises questions about their use in stabilizing bulk, three-dimensional (3D) perovskite solar cells through 3D/2D interfaces.
564. Excited-State Transient Chemistry of Rubrene: A Whole Story Jeffrey T. DuBose, Gábor Szabó, Jishnudas Chakkamalayath, and Prashant V. Kamat J. Phys. Chem. A. 2022, 126, 40, 11907–11914
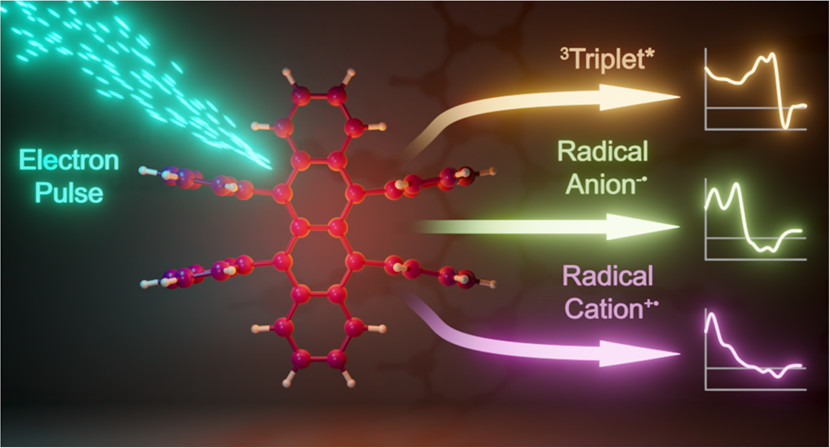 The ability to manipulate low-energy triplet excited states into higher-energy emissive singlet states, a process known as photon upconversion (UC), has potential applications in bioimaging, photocatalysis, and in increasing the efficiency of solar cells. However, the overall UC mechanism is complex and can involve many intermediate states, especially when semiconductors such as lead halide perovskites are used to sensitize the required triplet states. Using a combination of pulse radiolytic and electrochemical techniques, we have now explored the transient features of rubrene─a commonly employed triplet annihilator in UC systems. The rubrene triplet, radical anion, and radical cation species yield unique spectra that can serve as spectral fingerprints to distinguish between transient species formed during UC processes. Using detailed kinetic studies, we have succeeded in establishing that the rubrene triplets are susceptible to self-quenching (kquench = 3.6 × 108 M-1 s-1), and as the triplets decay, an additional transient feature is observed in the transient absorption spectra. This new feature indicates a net electron transfer process occurs to form the radical cation and anion as the triplets recombine. Taken together, this work provides a comprehensive picture of the excited state and transient features of rubrene and will be crucial for understanding the mechanism(s) of photon upconversion systems.
The ability to manipulate low-energy triplet excited states into higher-energy emissive singlet states, a process known as photon upconversion (UC), has potential applications in bioimaging, photocatalysis, and in increasing the efficiency of solar cells. However, the overall UC mechanism is complex and can involve many intermediate states, especially when semiconductors such as lead halide perovskites are used to sensitize the required triplet states. Using a combination of pulse radiolytic and electrochemical techniques, we have now explored the transient features of rubrene─a commonly employed triplet annihilator in UC systems. The rubrene triplet, radical anion, and radical cation species yield unique spectra that can serve as spectral fingerprints to distinguish between transient species formed during UC processes. Using detailed kinetic studies, we have succeeded in establishing that the rubrene triplets are susceptible to self-quenching (kquench = 3.6 × 108 M-1 s-1), and as the triplets decay, an additional transient feature is observed in the transient absorption spectra. This new feature indicates a net electron transfer process occurs to form the radical cation and anion as the triplets recombine. Taken together, this work provides a comprehensive picture of the excited state and transient features of rubrene and will be crucial for understanding the mechanism(s) of photon upconversion systems.
563. Metal Cocatalyst Dictates Electron Transfer in Ag-Decorated MoS2 Nanosheets Bo-An Chen, Sylwia Ptasinska, and Prashant V. Kamat J. Phys. Chem. C. 2022, 126, 29, 11907–11914
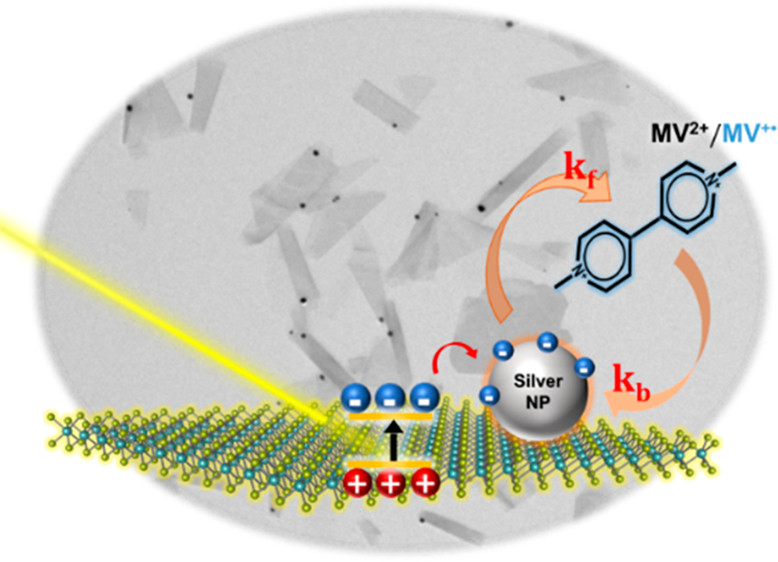 Two-dimensional transition-metal dichalcogenides such as atomically thin MoS2 nanosheets are useful as low-cost solar energy conversion materials. Colloidally stable few-layer and monolayer MoS2 nanosheets in dimethylformamide were prepared via an electrochemically assisted liquid phase exfoliation approach. These nanosheets were further modified with Ag nanoparticles by using photocatalytic reduction of Ag+ ions. An MV2+/MV•+ redox couple was employed as a probe to determine the rate constants of photoinduced forward electron transfer (kf) and dark back electron transfer (kb) processes to establish the role of Ag cocatalyst in promoting photocatalytic reduction. The competition between these two processes dictates the buildup of a steady-state concentration of the reduction product (MV•+) under visible-light irradiation. The kf and kb rate constants increase with increasing Ag nanoparticle loading but with different dependencies, yielding a maximum reduction efficiency of 4.4%. At higher Ag loadings, the reduction yield decreases as back electron transfer dominates over the forward electron transfer process. Establishing the role of noble metal cocatalyst in the photoinduced charge transfer processes of Ag-MoS2 hybrid composites offers design strategies to maximize the photocatalytic performance of semiconductor–metal heterostructures.
Two-dimensional transition-metal dichalcogenides such as atomically thin MoS2 nanosheets are useful as low-cost solar energy conversion materials. Colloidally stable few-layer and monolayer MoS2 nanosheets in dimethylformamide were prepared via an electrochemically assisted liquid phase exfoliation approach. These nanosheets were further modified with Ag nanoparticles by using photocatalytic reduction of Ag+ ions. An MV2+/MV•+ redox couple was employed as a probe to determine the rate constants of photoinduced forward electron transfer (kf) and dark back electron transfer (kb) processes to establish the role of Ag cocatalyst in promoting photocatalytic reduction. The competition between these two processes dictates the buildup of a steady-state concentration of the reduction product (MV•+) under visible-light irradiation. The kf and kb rate constants increase with increasing Ag nanoparticle loading but with different dependencies, yielding a maximum reduction efficiency of 4.4%. At higher Ag loadings, the reduction yield decreases as back electron transfer dominates over the forward electron transfer process. Establishing the role of noble metal cocatalyst in the photoinduced charge transfer processes of Ag-MoS2 hybrid composites offers design strategies to maximize the photocatalytic performance of semiconductor–metal heterostructures.
562. Energy Versus Electron Transfer: Managing Excited-State Interactions in Perovskite Nanocrystal–Molecular Hybrids (Focus Review) Jeffrey T. DuBose and Prashant V. Kamat Chem. Rev. 2022, 122, 15, 12475–12494
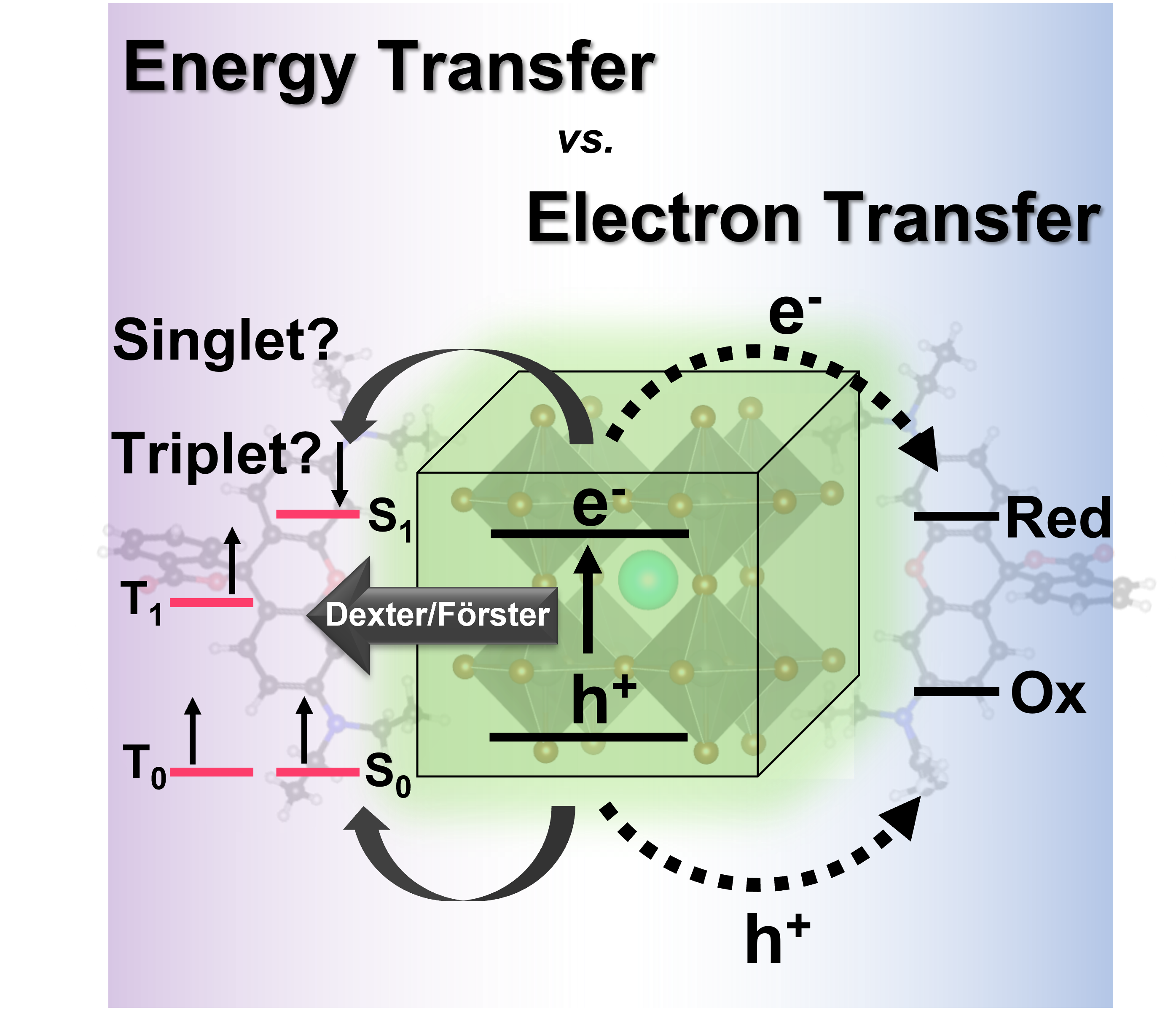 Energy and electron transfer processes in light harvesting assemblies dictate the outcome of the overall light energy conversion process. Halide perovskite nanocrystals such as CsPbBr3 with relatively high emission yield and strong light absorption can transfer singlet and triplet energy to surface-bound acceptor molecules. They can also induce photocatalytic reduction and oxidation by selectively transferring electrons and holes across the nanocrystal interface. This perspective discusses key factors dictating these excited-state pathways in perovskite nanocrystals and the fundamental differences between energy and electron transfer processes. Spectroscopic methods to decipher between these complex photoinduced pathways are presented. A basic understanding of the fundamental differences between the two excited deactivation processes (charge and energy transfer) and ways to modulate them should enable design of more efficient light harvesting assemblies with semiconductor and molecular systems.
Energy and electron transfer processes in light harvesting assemblies dictate the outcome of the overall light energy conversion process. Halide perovskite nanocrystals such as CsPbBr3 with relatively high emission yield and strong light absorption can transfer singlet and triplet energy to surface-bound acceptor molecules. They can also induce photocatalytic reduction and oxidation by selectively transferring electrons and holes across the nanocrystal interface. This perspective discusses key factors dictating these excited-state pathways in perovskite nanocrystals and the fundamental differences between energy and electron transfer processes. Spectroscopic methods to decipher between these complex photoinduced pathways are presented. A basic understanding of the fundamental differences between the two excited deactivation processes (charge and energy transfer) and ways to modulate them should enable design of more efficient light harvesting assemblies with semiconductor and molecular systems.
This Account discusses recent results that point to the specific role of hole trapping in phase segregation. Interestingly, generation of holes through above-band-gap excitation or through electrochemical injection increases ion migration and leads to phase segregation. The thermodynamic and redox properties of halide perovskites provide a strong driving force for hole trapping and oxidation of iodide species in MHPs. However, mobile halide species within the perovskite lattice take time to migrate and generate halide-rich domains. When in contact with a nonpolar solvent, the migration of iodine species is further extended to expulsion of iodine from the perovskite film. Thus, the mobility of halides and their susceptibility to hole-induced oxidation play a crucial role in determining the long-term stability of metal halide perovskites. Strategies to gain kinetic control over ion migration to slow phase segregation are needed to overcome these hurdles and achieve stable mixed halide perovskites. Modification of the perovskite composition through introduction of different cations or halide ions, or introduction of low-dimensional perovskite phases may suppress phase segregation. Thus, in achieving stability and improving the efficiency of perovskite solar cells and light emitting devices with minimal impacts, suppression of segregation remains the key factor..
561. Hole Trapping in Halide Perovskites Induces Phase Segregation (Review) Jeffrey T. DuBose and Prashant V. Kamat Acc. Mater. Res. 2022, 3, 7, 761–771
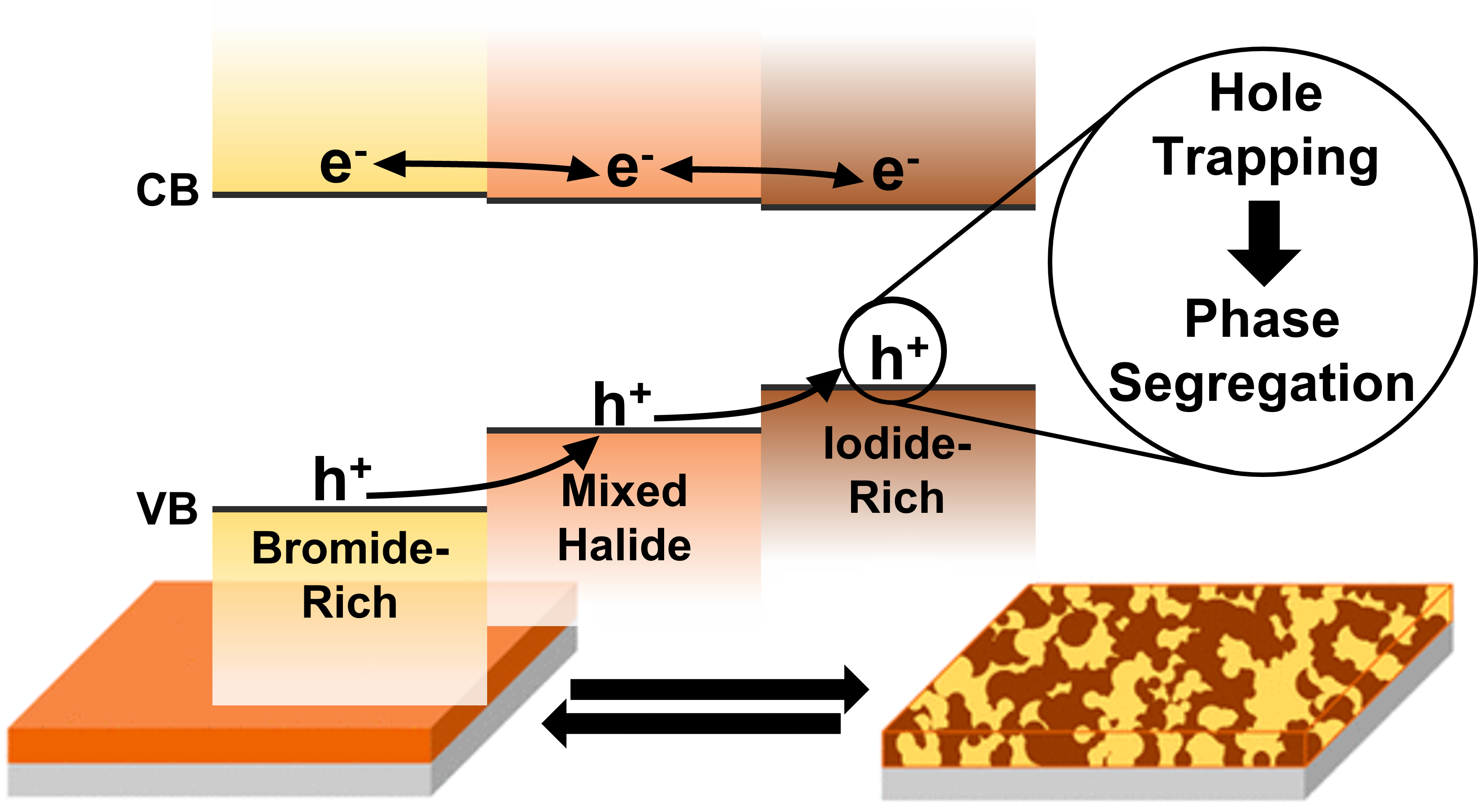 Metal halide perovskites have garnered a great deal of attention for their applications in photovoltaics, LEDs, and radiation detection. The ease of solution processing high-quality perovskite semiconductors with large absorption coefficients and tolerance to native defects is decidedly attractive. Additionally, the ability to precisely tune the band gap of halide perovskites through compositional alloying of the halide ion is of particular interest for a range of applications, especially for tandem solar cells. However, under steady state light irradiation, an initially homogeneous mixed halide perovskite (MHP) will form local domains that are rich in one halide ion (e.g., Br or I). This light-induced phase segregation in MHPs forms iodide-rich domains that act as charge carrier traps and lowers the efficiency of perovskite-based devices. Thus, phase segregation poses a serious challenge to the implementation of MHPs in real-world device settings. Interestingly, when a phase segregated MHP film is placed in the dark, entropic driving forces become dominant and the segregated perovskite remixes and returns to its initially homogeneous state. Several key mechanistic details of phase segregation have been elucidated over the years. However, there are still aspects of halide segregation that are not clear, and there is ongoing debate in the literature as to what are the key factors that contribute to the mechanism.
Metal halide perovskites have garnered a great deal of attention for their applications in photovoltaics, LEDs, and radiation detection. The ease of solution processing high-quality perovskite semiconductors with large absorption coefficients and tolerance to native defects is decidedly attractive. Additionally, the ability to precisely tune the band gap of halide perovskites through compositional alloying of the halide ion is of particular interest for a range of applications, especially for tandem solar cells. However, under steady state light irradiation, an initially homogeneous mixed halide perovskite (MHP) will form local domains that are rich in one halide ion (e.g., Br or I). This light-induced phase segregation in MHPs forms iodide-rich domains that act as charge carrier traps and lowers the efficiency of perovskite-based devices. Thus, phase segregation poses a serious challenge to the implementation of MHPs in real-world device settings. Interestingly, when a phase segregated MHP film is placed in the dark, entropic driving forces become dominant and the segregated perovskite remixes and returns to its initially homogeneous state. Several key mechanistic details of phase segregation have been elucidated over the years. However, there are still aspects of halide segregation that are not clear, and there is ongoing debate in the literature as to what are the key factors that contribute to the mechanism.
This Account discusses recent results that point to the specific role of hole trapping in phase segregation. Interestingly, generation of holes through above-band-gap excitation or through electrochemical injection increases ion migration and leads to phase segregation. The thermodynamic and redox properties of halide perovskites provide a strong driving force for hole trapping and oxidation of iodide species in MHPs. However, mobile halide species within the perovskite lattice take time to migrate and generate halide-rich domains. When in contact with a nonpolar solvent, the migration of iodine species is further extended to expulsion of iodine from the perovskite film. Thus, the mobility of halides and their susceptibility to hole-induced oxidation play a crucial role in determining the long-term stability of metal halide perovskites. Strategies to gain kinetic control over ion migration to slow phase segregation are needed to overcome these hurdles and achieve stable mixed halide perovskites. Modification of the perovskite composition through introduction of different cations or halide ions, or introduction of low-dimensional perovskite phases may suppress phase segregation. Thus, in achieving stability and improving the efficiency of perovskite solar cells and light emitting devices with minimal impacts, suppression of segregation remains the key factor..
560. Efficacy of Perovskite Photocatalysis: Challenges to Overcome (Perspective) Jeffrey T. DuBose and Prashant V. Kamat ACS Energy Lett. 2022, 7, 6, 1994–2011
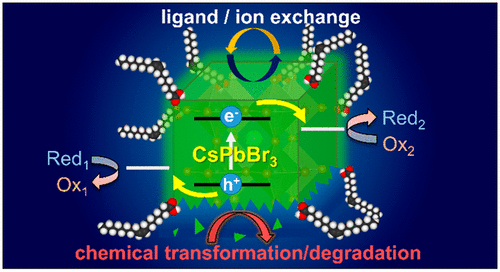 Having gained prominence in the design of high-efficiency solar cells, metal halide perovskites have taken center stage in photocatalysis. Despite the demonstrations of photocatalytic activity in inducing reduction and oxidation processes through visible-light excitation, the long-term stability of CsPbBr3 and other perovskite nanocrystals and nanostructures still poses a challenge. This Perspective discusses how factors such as instability arising from labile ligands, ease of halide exchange, halide mobility under photoirradiation, chemical and morphological transformations in polar solvents, and difficulty in designing heterostructures pose major hurdles in developing halide perovskites as photocatalysts. The key examples of organic and inorganic transformations as well as interfacial charge-transfer processes discussed here present some unique features of perovskite photocatalysts and highlight the complexity in their utilization for future chemical conversion processes. Strategies to protect the perovskite surface are needed to maintain the required long-term stability in practical applications.
Having gained prominence in the design of high-efficiency solar cells, metal halide perovskites have taken center stage in photocatalysis. Despite the demonstrations of photocatalytic activity in inducing reduction and oxidation processes through visible-light excitation, the long-term stability of CsPbBr3 and other perovskite nanocrystals and nanostructures still poses a challenge. This Perspective discusses how factors such as instability arising from labile ligands, ease of halide exchange, halide mobility under photoirradiation, chemical and morphological transformations in polar solvents, and difficulty in designing heterostructures pose major hurdles in developing halide perovskites as photocatalysts. The key examples of organic and inorganic transformations as well as interfacial charge-transfer processes discussed here present some unique features of perovskite photocatalysts and highlight the complexity in their utilization for future chemical conversion processes. Strategies to protect the perovskite surface are needed to maintain the required long-term stability in practical applications.
559. Managing photoinduced electron transfer in AgInS2–CdS heterostructures Anthony Kipkorir and Prashant V. Kamat J. Chem. Phys. 156, 174703 (2022)
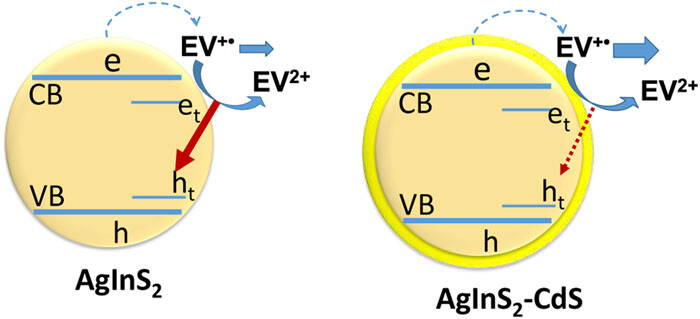 Ternary semiconductors such as AgInS, with their interesting photocatalytic properties, can serve as building blocks to design light harvesting assemblies. The intraband transitions created by the metal ions extend the absorption well beyond the bandgap transition. The interfacial electron transfer of AgInS with surface bound ethyl viologen under bandgap and sub-bandgap irradiation as probed by steady state photolysis and transient absorption spectroscopy offers new insights into the participation of conduction band and trapped electrons. Capping AgInS with CdS shifts emission maximum to the blue and increases the emission yield as the surface defects are remediated. CdS capping also promotes charge separation as evident from the efficiency of electron transfer to ethyl viologen, which increased from 14% to 29%. The transient absorption measurements that elucidate the kinetic aspects of electron transfer processes in AgInS and CdS capped AgInS are presented. The improved performance of CdS capped AgInS offers new opportunities to employ them as photocatalysts.
Ternary semiconductors such as AgInS, with their interesting photocatalytic properties, can serve as building blocks to design light harvesting assemblies. The intraband transitions created by the metal ions extend the absorption well beyond the bandgap transition. The interfacial electron transfer of AgInS with surface bound ethyl viologen under bandgap and sub-bandgap irradiation as probed by steady state photolysis and transient absorption spectroscopy offers new insights into the participation of conduction band and trapped electrons. Capping AgInS with CdS shifts emission maximum to the blue and increases the emission yield as the surface defects are remediated. CdS capping also promotes charge separation as evident from the efficiency of electron transfer to ethyl viologen, which increased from 14% to 29%. The transient absorption measurements that elucidate the kinetic aspects of electron transfer processes in AgInS and CdS capped AgInS are presented. The improved performance of CdS capped AgInS offers new opportunities to employ them as photocatalysts.
558. Photoinduced Transformation of Cs2Au2Br6 into CsPbBr3 Nanocrystals Jishnudas Chakkamalayath, Gregory V. Hartland, and Prashant V. Kamat J. Phys. Chem. Lett. 2022, 13, 13, 2921–2927
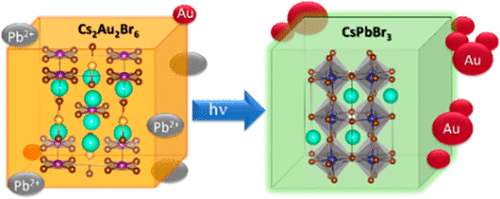 Lead-free halide double perovskites offer an environmentally friendly alternative to lead halide perovskites for designing optoelectronic solar cell devices. One simple approach to synthesize such double halide perovskites is through metal ion exchange. CsPbBr3 nanocrystals undergo exchange of Pb2+ with Au(I)/Au(III) to form double perovskite Cs2Au2Br6. When excited, a majority of the charge carriers undergo quick recombination in contrast to long-lived charge carries of excited CsPbBr3 nanocrystals. This metal ion exchange process is reversible as one can regenerate CsPbBr3 by adding excess PbBr2 to the suspension. Interestingly, when subjected to visible light irradiation, Cs2Au2Br6 nanocrystals eject reduced Au from the lattice as evidenced from the formation of larger gold nanoparticles. The presence of residual Pb2+ ions in the suspension restores the original CsPbBr3 composition. The results presented here provide insight into the dynamic nature of Au within the perovskite lattice under both chemical and light stimuli.
Lead-free halide double perovskites offer an environmentally friendly alternative to lead halide perovskites for designing optoelectronic solar cell devices. One simple approach to synthesize such double halide perovskites is through metal ion exchange. CsPbBr3 nanocrystals undergo exchange of Pb2+ with Au(I)/Au(III) to form double perovskite Cs2Au2Br6. When excited, a majority of the charge carriers undergo quick recombination in contrast to long-lived charge carries of excited CsPbBr3 nanocrystals. This metal ion exchange process is reversible as one can regenerate CsPbBr3 by adding excess PbBr2 to the suspension. Interestingly, when subjected to visible light irradiation, Cs2Au2Br6 nanocrystals eject reduced Au from the lattice as evidenced from the formation of larger gold nanoparticles. The presence of residual Pb2+ ions in the suspension restores the original CsPbBr3 composition. The results presented here provide insight into the dynamic nature of Au within the perovskite lattice under both chemical and light stimuli.
557. Do Sacrificial Donors Donate H2 in Photocatalysis? (Viewpoint) Federica Costantino and Prashant V. Kamat ACS Energy Lett. 2022, 7, 1, 242–246
 Generation of solar fuels using a semiconductor photocatalyst continues to be a popular topic in light–energy conversion. Semiconductor-assisted photoelectrolysis usually includes water (or H+) reduction to produce H2 or CO2 reduction to produce value-added chemicals. Significant strides have been made in recent years to design new photocatalysts and obtain mechanistic insights into the interfacial charge-transfer processes in semiconductor particle systems. However, semiconductors such as metal chalcogenides (e.g., CdS) are susceptible to hole-induced oxidation (anodic corrosion) when employed as photocatalysts for H2 generation. Sacrificial donors such as alcohols, amines, ascorbic acid, and EDTA are commonly employed to scavenge the photogenerated holes and, thus, maintain photocatalyst stability. The product formed in such a process (e.g., H2) is often assumed to arise exclusively from the reduction process. Because of this popular belief, the direct involvement of sacrificial donors in the net accumulation of the product often goes unchecked.
Generation of solar fuels using a semiconductor photocatalyst continues to be a popular topic in light–energy conversion. Semiconductor-assisted photoelectrolysis usually includes water (or H+) reduction to produce H2 or CO2 reduction to produce value-added chemicals. Significant strides have been made in recent years to design new photocatalysts and obtain mechanistic insights into the interfacial charge-transfer processes in semiconductor particle systems. However, semiconductors such as metal chalcogenides (e.g., CdS) are susceptible to hole-induced oxidation (anodic corrosion) when employed as photocatalysts for H2 generation. Sacrificial donors such as alcohols, amines, ascorbic acid, and EDTA are commonly employed to scavenge the photogenerated holes and, thus, maintain photocatalyst stability. The product formed in such a process (e.g., H2) is often assumed to arise exclusively from the reduction process. Because of this popular belief, the direct involvement of sacrificial donors in the net accumulation of the product often goes unchecked.
556. Transformation of Perovskite Nanoplatelets to Large Nanostructures Driven by Solvent Polarity Jeffrey T. DuBose, Andrew Christy, Jishnudas Chakkamalayath, and Prashant V. Kamat ACS Materials Lett. 2022, 4, XXX, 93–101
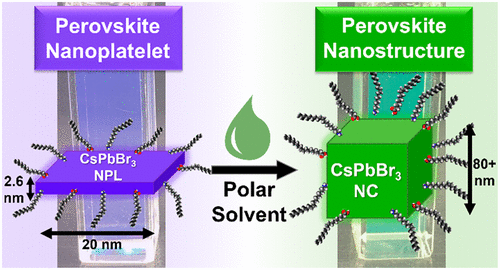 Perovskite nanoplatelets have potential use in optoelectronic and photocatalytic applications because of the enhanced quantum confinement they experience. However, often ignored is the effect of solvent polarity on the stability of these nanoplatelets. When perovskite nanoplatelets are exposed to small amounts (<1 vol %) of mildly polar solvents such as acetonitrile, the particles rapidly grow to larger nanostructures. Their quantum confinement is lost as they grow from uniform 2.6 nm thick particles to large nanostructures ~85 nm in size. This ripening brings characteristic red-shifts in the absorption and photoluminescence of the particles as they transform. Using methyl acetate as a model polar solvent, we succeeded in establishing factors that control ligand desorption and lowering of the activation energy for ripening. These results highlight the challenges in using these quantum-confined nanoplatelets in applications such as photocatalysis, where polar solvents and/or intermediates may be unavoidable.
Perovskite nanoplatelets have potential use in optoelectronic and photocatalytic applications because of the enhanced quantum confinement they experience. However, often ignored is the effect of solvent polarity on the stability of these nanoplatelets. When perovskite nanoplatelets are exposed to small amounts (<1 vol %) of mildly polar solvents such as acetonitrile, the particles rapidly grow to larger nanostructures. Their quantum confinement is lost as they grow from uniform 2.6 nm thick particles to large nanostructures ~85 nm in size. This ripening brings characteristic red-shifts in the absorption and photoluminescence of the particles as they transform. Using methyl acetate as a model polar solvent, we succeeded in establishing factors that control ligand desorption and lowering of the activation energy for ripening. These results highlight the challenges in using these quantum-confined nanoplatelets in applications such as photocatalysis, where polar solvents and/or intermediates may be unavoidable.
2021
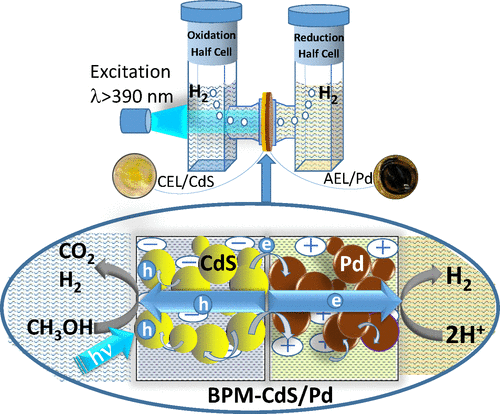
555. A Bipolar CdS/Pd Photocatalytic Membrane for Selective Segregation of Reduction and Oxidation Processes Federica Costantino, Luca Gavioli, and Prashant V. Kamat ACS Phys. Chem Au 2022, 2, 2, 89–97
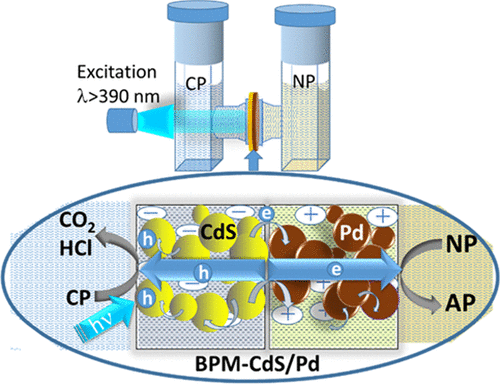 A photocatalytically active bipolar membrane consisting of a CdS photocatalyst and Pd electrocatalyst has been constructed to carry out environmentally relevant oxidation and reduction processes. The ion exchange property of a bipolar membrane (BPM) has allowed us to load the CdS photocatalyst on one side and Pd electrocatalyst on the other side. By inserting the photocatalytic BPM-CdS/Pd membrane between the two compartments of an H-cell, we can separate the reduction and oxidation processes. Following visible light excitation of CdS in the BPM-CdS/Pd membrane, we can induce vectorial electron transfer from CdS to Pd and to an electron acceptor (4-nitrophenol). The holes generated at CdS are scavenged by ethanol or 4-chlorophenol. The photocatalytic reduction rate dependence on the Pd loading in the membrane as well as its effect on modulating the rates of electron and hole transfer processes are discussed. The design of a semiconductor and metal loaded membrane paves the way for improving selectivity and efficiency of photocatalytic processes.
A photocatalytically active bipolar membrane consisting of a CdS photocatalyst and Pd electrocatalyst has been constructed to carry out environmentally relevant oxidation and reduction processes. The ion exchange property of a bipolar membrane (BPM) has allowed us to load the CdS photocatalyst on one side and Pd electrocatalyst on the other side. By inserting the photocatalytic BPM-CdS/Pd membrane between the two compartments of an H-cell, we can separate the reduction and oxidation processes. Following visible light excitation of CdS in the BPM-CdS/Pd membrane, we can induce vectorial electron transfer from CdS to Pd and to an electron acceptor (4-nitrophenol). The holes generated at CdS are scavenged by ethanol or 4-chlorophenol. The photocatalytic reduction rate dependence on the Pd loading in the membrane as well as its effect on modulating the rates of electron and hole transfer processes are discussed. The design of a semiconductor and metal loaded membrane paves the way for improving selectivity and efficiency of photocatalytic processes.
554. Directing Energy Transfer in Halide Perovskite–Chromophore Hybrid Assemblies Jeffrey T. DuBose and Prashant V. Kamat J. Am. Chem. Soc. 2021, 143, 45, 19214–19223
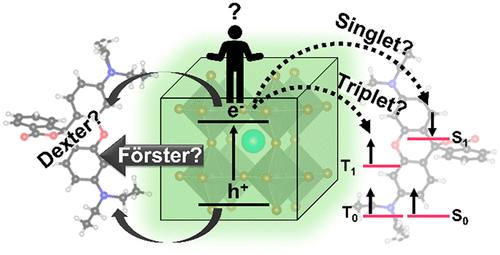 Directing the flow of energy and the nature of the excited states that are produced in nanocrystal–chromophore hybrid assemblies is crucial for realizing their photocatalytic and optoelectronic applications. Using a combination of steady-state and time-resolved absorption and photoluminescence (PL) experiments, we have probed the excited-state interactions in the CsPbBr3–Rhodamine B (RhB) hybrid assembly. PL studies reveal quenching of the CsPbBr3 emission with a concomitant enhancement of the fluorescence of RhB, indicating a singlet-energy-transfer mechanism. Transient absorption spectroscopy shows that this energy transfer occurs on the ~200 ps time scale. To understand whether the energy transfer occurs through a Förster or Dexter mechanism, we leveraged facile halide-exchange reactions to tune the optical properties of the donor CsPbBr3 by alloying with chloride. This allowed us to tune the spectral overlap between the donor CsPb(Br1–xClx)3 emission and acceptor RhB absorption. For CsPbBr3-RhB, the rate constant for energy transfer (kET) agrees well with Förster theory, whereas alloying with chloride to produce chloride-rich CsPb(Br1–xClx)3 favors a Dexter mechanism. These results highlight the importance of optimizing both the donor and acceptor properties to design light-harvesting assemblies that employ energy transfer. The ease of tuning optical properties through halide exchange of the nanocrystal donor provides a unique platform for studying and tailoring excited-state interactions in perovskite–chromophore assemblies.
Directing the flow of energy and the nature of the excited states that are produced in nanocrystal–chromophore hybrid assemblies is crucial for realizing their photocatalytic and optoelectronic applications. Using a combination of steady-state and time-resolved absorption and photoluminescence (PL) experiments, we have probed the excited-state interactions in the CsPbBr3–Rhodamine B (RhB) hybrid assembly. PL studies reveal quenching of the CsPbBr3 emission with a concomitant enhancement of the fluorescence of RhB, indicating a singlet-energy-transfer mechanism. Transient absorption spectroscopy shows that this energy transfer occurs on the ~200 ps time scale. To understand whether the energy transfer occurs through a Förster or Dexter mechanism, we leveraged facile halide-exchange reactions to tune the optical properties of the donor CsPbBr3 by alloying with chloride. This allowed us to tune the spectral overlap between the donor CsPb(Br1–xClx)3 emission and acceptor RhB absorption. For CsPbBr3-RhB, the rate constant for energy transfer (kET) agrees well with Förster theory, whereas alloying with chloride to produce chloride-rich CsPb(Br1–xClx)3 favors a Dexter mechanism. These results highlight the importance of optimizing both the donor and acceptor properties to design light-harvesting assemblies that employ energy transfer. The ease of tuning optical properties through halide exchange of the nanocrystal donor provides a unique platform for studying and tailoring excited-state interactions in perovskite–chromophore assemblies.
553. CsPbBr3–CdS heterostructure: stabilizing perovskite nanocrystals for photocatalysis Anthony Kipkorir, Jeffrey DuBose, Junsang Cho, and Prashant V. Kamat Chem. Sci. 2021,12, 14815-14825
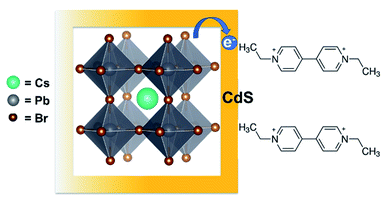 The instability of cesium lead bromide (CsPbBr3) nanocrystals (NCs) in polar solvents has hampered their use in photocatalysis. We have now succeeded in synthesizing CsPbBr3–CdS heterostructures with improved stability and photocatalytic performance. While the CdS deposition provides solvent stability, the parent CsPbBr3 in the heterostructure harvests photons to generate charge carriers. This heterostructure exhibits longer emission lifetime (τave = 47 ns) than pristine CsPbBr3 (τave = 7 ns), indicating passivation of surface defects. We employed ethyl viologen (EV2+) as a probe molecule to elucidate excited state interactions and interfacial electron transfer of CsPbBr3–CdS NCs in toluene/ethanol mixed solvent. The electron transfer rate constant as obtained from transient absorption spectroscopy was 9.5 × 1010 s−1 and the quantum efficiency of ethyl viologen reduction (ΦEV+˙) was found to be 8.4% under visible light excitation. The Fermi level equilibration between CsPbBr3–CdS and EV2+/EV+˙ redox couple has allowed us to estimate the apparent conduction band energy of the heterostructure as −0.365 V vs. NHE. The insights into effective utilization of perovskite nanocrystals built around a quasi-type II heterostructures pave the way towards effective utilization in photocatalytic reduction and oxidation processes.
The instability of cesium lead bromide (CsPbBr3) nanocrystals (NCs) in polar solvents has hampered their use in photocatalysis. We have now succeeded in synthesizing CsPbBr3–CdS heterostructures with improved stability and photocatalytic performance. While the CdS deposition provides solvent stability, the parent CsPbBr3 in the heterostructure harvests photons to generate charge carriers. This heterostructure exhibits longer emission lifetime (τave = 47 ns) than pristine CsPbBr3 (τave = 7 ns), indicating passivation of surface defects. We employed ethyl viologen (EV2+) as a probe molecule to elucidate excited state interactions and interfacial electron transfer of CsPbBr3–CdS NCs in toluene/ethanol mixed solvent. The electron transfer rate constant as obtained from transient absorption spectroscopy was 9.5 × 1010 s−1 and the quantum efficiency of ethyl viologen reduction (ΦEV+˙) was found to be 8.4% under visible light excitation. The Fermi level equilibration between CsPbBr3–CdS and EV2+/EV+˙ redox couple has allowed us to estimate the apparent conduction band energy of the heterostructure as −0.365 V vs. NHE. The insights into effective utilization of perovskite nanocrystals built around a quasi-type II heterostructures pave the way towards effective utilization in photocatalytic reduction and oxidation processes.
552. Photoinduced Halide Segregation in Ruddlesden–Popper 2D Mixed Halide Perovskite Films Junsang Cho, Preethi S. Mathew, Jeffrey T. DuBose, Prashant V. Kamat Adv. Mater. 2021, 2105585.
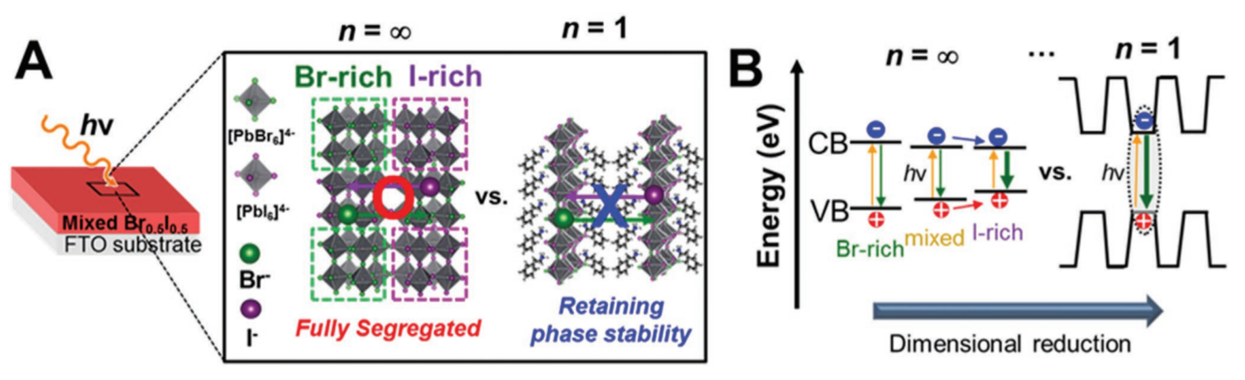 2D lead halide perovskites, which exhibit bandgap tunability and increased chemical stability, have been found to be useful for designing optoelectronic devices. Reducing dimensionality with decreasing number of layers (n = 10–1) also imparts resistance to light-induced ion migration as seen from the halide ion segregation and dark recovery in mixed halide (Br:I = 50:50) perovskite films. The light-induced halide ion segregation efficiency, as determined from difference absorbance spectra, decreases from 20% to 1% as the dimensionality is decreased for 2D perovskite film from n = 10 to 1. The segregation rate constant (ksegregation), which decreases from 5.9 × 10−3 s−1 (n = 10) to 3.6 × 10−4 s−1 (n = 1), correlates well with nearly an order of magnitude decrease observed in charge-carrier lifetime (τaverage = 233 ps for n = 10 vs τavg = 27 ps for n = 1). The tightly bound excitons in 2D perovskites make charge separation less probable, which in turn decreases the halide mobility and resulting phase segregation. The importance of controlling the dimensionality of the 2D architecture in suppressing halide ion mobility is discussed.
2D lead halide perovskites, which exhibit bandgap tunability and increased chemical stability, have been found to be useful for designing optoelectronic devices. Reducing dimensionality with decreasing number of layers (n = 10–1) also imparts resistance to light-induced ion migration as seen from the halide ion segregation and dark recovery in mixed halide (Br:I = 50:50) perovskite films. The light-induced halide ion segregation efficiency, as determined from difference absorbance spectra, decreases from 20% to 1% as the dimensionality is decreased for 2D perovskite film from n = 10 to 1. The segregation rate constant (ksegregation), which decreases from 5.9 × 10−3 s−1 (n = 10) to 3.6 × 10−4 s−1 (n = 1), correlates well with nearly an order of magnitude decrease observed in charge-carrier lifetime (τaverage = 233 ps for n = 10 vs τavg = 27 ps for n = 1). The tightly bound excitons in 2D perovskites make charge separation less probable, which in turn decreases the halide mobility and resulting phase segregation. The importance of controlling the dimensionality of the 2D architecture in suppressing halide ion mobility is discussed.
551. Light Induced Processes in CsPbBr3–Au Hybrid Nanocrystals: Electron Transfer and Expulsion of Au Jishnudas Chakkamalayath, Gregory V. Hartland, and Prashant V. Kamat J. Phys. Chem. C 2021, 125, 32, 17881–17889.
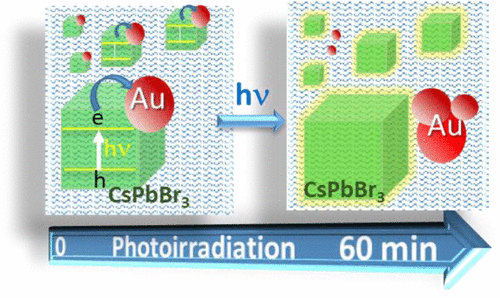 Semiconductor–metal heterostructures such as CsPbBr3–Au are useful in photocatalysis. When Au nanoparticles are deposited on the CsPbBr3 nanocrystal surface, they efficiently quench the photoluminescence of the semiconductor. This process has been studied by femtosecond transient absorption spectroscopy measurements, which indicate that electron transfer to the Au nanoparticles occurs from both hot and relaxed electrons in the conduction band of CsPbBr3. The electron transfer rate constant is much larger for the hot electrons compared to the relaxed electrons. Under steady state photoirradiation of CsPbBr3–Au heterostructure, the photogenerated electrons from the excited CsPbBr3 nanocrystals continue to charge the Au nanoparticles. After sufficient irradiation, the gold nanoparticles dissociate from the CsPbBr3 surface and aggregate into larger size gold nanoparticles. The expulsion of gold nanoparticles restores the original luminescence behavior of CsPbBr3 nanocrystals. The spectroscopic and morphological studies provide insight into the expulsion of gold nanoparticles in photoirradiated CsPbBr3–Au heterostructures.
Semiconductor–metal heterostructures such as CsPbBr3–Au are useful in photocatalysis. When Au nanoparticles are deposited on the CsPbBr3 nanocrystal surface, they efficiently quench the photoluminescence of the semiconductor. This process has been studied by femtosecond transient absorption spectroscopy measurements, which indicate that electron transfer to the Au nanoparticles occurs from both hot and relaxed electrons in the conduction band of CsPbBr3. The electron transfer rate constant is much larger for the hot electrons compared to the relaxed electrons. Under steady state photoirradiation of CsPbBr3–Au heterostructure, the photogenerated electrons from the excited CsPbBr3 nanocrystals continue to charge the Au nanoparticles. After sufficient irradiation, the gold nanoparticles dissociate from the CsPbBr3 surface and aggregate into larger size gold nanoparticles. The expulsion of gold nanoparticles restores the original luminescence behavior of CsPbBr3 nanocrystals. The spectroscopic and morphological studies provide insight into the expulsion of gold nanoparticles in photoirradiated CsPbBr3–Au heterostructures.
550. State of the Art and Prospects for Halide Perovskite Nanocrystals Amrita Dey, Junzhi Ye, Apurba De, Elke Debroye, Seung Kyun Ha, Eva Bladt, Anuraj S. Kshirsagar, Ziyu Wang, Jun Yin, Yue Wang, Li Na Quan, Fei Yan, Mengyu Gao, Xiaoming Li, Javad Shamsi, Tushar Debnath, Muhan Cao, Manuel A. Scheel, Sudhir Kumar, Julian A. Steele, Marina Gerhard, Lata Chouhan, Ke Xu, Xian-gang Wu, Yanxiu Li, Yangning Zhang, Anirban Dutta, Chuang Han, Ilka Vincon, Andrey L. Rogach, Angshuman Nag, Anunay Samanta, Brian A. Korgel, Chih-Jen Shih, Daniel R. Gamelin, Dong Hee Son, Haibo Zeng, Haizheng Zhong, Handong Sun, Hilmi Volkan Demir, Ivan G. Scheblykin, Iván Mora-Seró, Jacek K. Stolarczyk, Jin Z. Zhang, Jochen Feldmann, Johan Hofkens, Joseph M. Luther, Julia Pérez-Prieto, Liang Li, Liberato Manna, Maryna I. Bodnarchuk, Maksym V. Kovalenko, Maarten B. J. Roeffaers, Narayan Pradhan, Omar F. Mohammed, Osman M. Bakr, Peidong Yang, Peter Müller-Buschbaum, Prashant V. Kamat, Qiaoliang Bao, Qiao Zhang, Roman Krahne, Raquel E. Galian, Samuel D. Stranks, Sara Bals, Vasudevanpillai Biju, William A. Tisdale, Yong Yan, Robert L. Z. Hoye, and Lakshminarayana Polavarapu ACS Nano 2021, 15, 7, 10775–10981
 Metal-halide perovskites have rapidly emerged as one of the most promising materials of the 21st century, with many exciting properties and great potential for a broad range of applications, from photovoltaics to optoelectronics and photocatalysis. The ease with which metal-halide perovskites can be synthesized in the form of brightly luminescent colloidal nanocrystals, as well as their tunable and intriguing optical and electronic properties, has attracted researchers from different disciplines of science and technology. In the last few years, there has been a significant progress in the shape-controlled synthesis of perovskite nanocrystals and understanding of their properties and applications. In this comprehensive review, researchers having expertise in different fields (chemistry, physics, and device engineering) of metal-halide perovskite nanocrystals have joined together to provide a state of the art overview and future prospects of metal-halide perovskite nanocrystal research.
Metal-halide perovskites have rapidly emerged as one of the most promising materials of the 21st century, with many exciting properties and great potential for a broad range of applications, from photovoltaics to optoelectronics and photocatalysis. The ease with which metal-halide perovskites can be synthesized in the form of brightly luminescent colloidal nanocrystals, as well as their tunable and intriguing optical and electronic properties, has attracted researchers from different disciplines of science and technology. In the last few years, there has been a significant progress in the shape-controlled synthesis of perovskite nanocrystals and understanding of their properties and applications. In this comprehensive review, researchers having expertise in different fields (chemistry, physics, and device engineering) of metal-halide perovskite nanocrystals have joined together to provide a state of the art overview and future prospects of metal-halide perovskite nanocrystal research.
549. Spacer Cations Dictate Photoinduced Phase Segregation in 2D Mixed Halide Perovskites Preethi S. Mathew, Jeffrey T. DuBose, Junsang Cho, and Prashant V. Kamat ACS Energy Lett. 2021, 6, 7, 2499–2501.
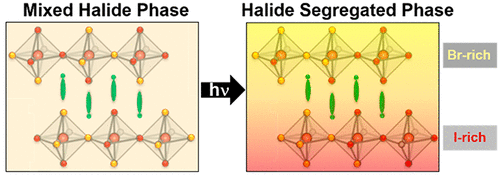 The spacer cation in two-dimensional (2D) Ruddlesden–Popper lead halide perovskites dictates photoinduced phase segregation. Halide ion segregation under steady-state irradiation of single-layer (n = 1) 2D perovskites is seen when butylammonium (BA) is used as the spacer cation, whereas it is essentially absent when BA is replaced with phenethylammonium (PEA).
The spacer cation in two-dimensional (2D) Ruddlesden–Popper lead halide perovskites dictates photoinduced phase segregation. Halide ion segregation under steady-state irradiation of single-layer (n = 1) 2D perovskites is seen when butylammonium (BA) is used as the spacer cation, whereas it is essentially absent when BA is replaced with phenethylammonium (PEA).
548. Vectorial Charge Transfer across Bipolar Membrane Loaded with CdS and Au Nanoparticles Manjeet Chhetri and Prashant V. Kamat J. Phys. Chem. C 2021, 125, 12, 6870–6876.
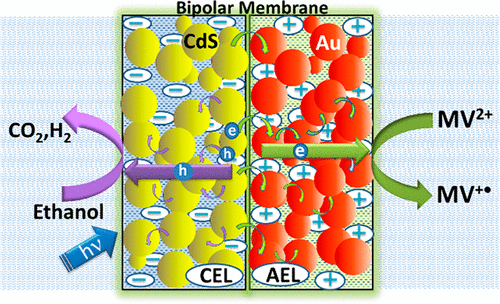 Bipolar membranes (BPMs) which consist of a cation exchange layer (CEL) and anion exchange layer (AEL) are quite effective as membranes in gas phase electrolyzers. However, such membranes can also serve as a host to embed photocatalysts and electrocatalysts. By selectively exchanging cations with Cd2+ and anions with AuCl4–, we were able to synthesize CdS and Au nanoparticles in CEL and AEL layers, respectively, through sequential chemical and photocatalytic reactions. Reacting Cd2+ with thioacetamide formed the CdS nanoparticles in CEL. The photogenerated electrons from CdS were then used to reduce AuCl4– in an H-cell configuration to produce Au nanoparticles in AEL and thus prepare a photocatalytically active BPM film (referred to as a CdS/BPM/Au film). Such a concerted design of BPM allows “vectorial” electron transfer between two layers of BPM leading to its transfer to an acceptor molecule (methyl viologen) in solution. Designing photocatalytically active BPM and understanding the vectorial electron flow between two separate ion-selective layers offer new opportunities in water splitting and CO2 reduction.
Bipolar membranes (BPMs) which consist of a cation exchange layer (CEL) and anion exchange layer (AEL) are quite effective as membranes in gas phase electrolyzers. However, such membranes can also serve as a host to embed photocatalysts and electrocatalysts. By selectively exchanging cations with Cd2+ and anions with AuCl4–, we were able to synthesize CdS and Au nanoparticles in CEL and AEL layers, respectively, through sequential chemical and photocatalytic reactions. Reacting Cd2+ with thioacetamide formed the CdS nanoparticles in CEL. The photogenerated electrons from CdS were then used to reduce AuCl4– in an H-cell configuration to produce Au nanoparticles in AEL and thus prepare a photocatalytically active BPM film (referred to as a CdS/BPM/Au film). Such a concerted design of BPM allows “vectorial” electron transfer between two layers of BPM leading to its transfer to an acceptor molecule (methyl viologen) in solution. Designing photocatalytically active BPM and understanding the vectorial electron flow between two separate ion-selective layers offer new opportunities in water splitting and CO2 reduction.
547. Modulation of Photoinduced Iodine Expulsion in Mixed Halide Perovskites with Electrochemical Bias Jeffrey T. DuBose, Preethi S. Mathew, Junsang Cho, Masaru Kuno, and Prashant V. Kamat J. Phys. Chem. Lett. 2021, 12, 10, 2615–2621.
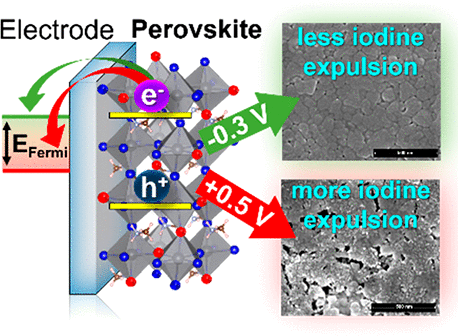 Hole trapping at iodine (I) sites in MAPbBr1.5I1.5 mixed halide perovskites (MHP) is responsible for iodine migration and its eventual expulsion into solution. We have now modulated the photoinduced iodine expulsion in MHP through an externally applied electrochemical bias. At positive potentials, electron extraction at TiO2/MHP interfaces becomes efficient, leading to hole buildup within MHP films. This improved charge separation, in turn, favors iodine migration as evident from the increased apparent rate constant of iodine expulsion (kexpulsion = 0.0030 s–1). Conversely, at negative potentials (−0.3 V vs Ag/AgCl) electron–hole recombination is facilitated within MHP, slowing down iodine expulsion by an order of magnitude (kexpulsion = 0.00018 s–1). The tuning of the EFermi level through external bias modulates electron extraction at the TiO2/MHP interface and indirectly controls the buildup of holes, ultimately inducing iodine migration/expulsion. Suppressing iodine migration in perovskite solar cells is important for attaining greater stability since they operate under internal electrical bias.
Hole trapping at iodine (I) sites in MAPbBr1.5I1.5 mixed halide perovskites (MHP) is responsible for iodine migration and its eventual expulsion into solution. We have now modulated the photoinduced iodine expulsion in MHP through an externally applied electrochemical bias. At positive potentials, electron extraction at TiO2/MHP interfaces becomes efficient, leading to hole buildup within MHP films. This improved charge separation, in turn, favors iodine migration as evident from the increased apparent rate constant of iodine expulsion (kexpulsion = 0.0030 s–1). Conversely, at negative potentials (−0.3 V vs Ag/AgCl) electron–hole recombination is facilitated within MHP, slowing down iodine expulsion by an order of magnitude (kexpulsion = 0.00018 s–1). The tuning of the EFermi level through external bias modulates electron extraction at the TiO2/MHP interface and indirectly controls the buildup of holes, ultimately inducing iodine migration/expulsion. Suppressing iodine migration in perovskite solar cells is important for attaining greater stability since they operate under internal electrical bias.
546. Halide Ion Migration in Perovskite Nanocrystals and Nanostructures (Review) Prashant V. Kamat and Masaru Kuno Acc. Chem. Res. 2021, 54, 3, 520–531
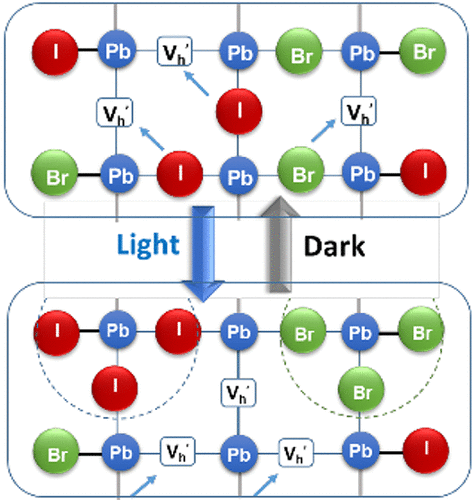 The optical and electronic properties of metal halide perovskites provide insight into the operation of solar cells as well as their long-term operational stability. Halide mobility in perovskite films is an important factor influencing solar cell performance. One can visualize halide ion migration through halide exchange between two nanocrystal suspensions or between physically paired films of two different metal halide perovskites. The ability to tune band gap by varying halide ratios (Cl:Br or Br:I) allows the synthesis of mixed halide perovskites with tailored absorption and emission across the entire visible spectrum. Interestingly, mixed halide (e.g., MAPb(Br0.5I0.5)3) films undergo phase segregation to form Br-rich and I-rich sites under steady state illumination. Upon halting illumination, segregated phases mix to restore original mixed halide compositions. Introducing multiple cations (Cs, formamidinium) at the A site or alloying with Cl greatly suppresses halide mobilities. Long-term irradiation of MAPb(Br0.5I0.5)3 films also cause expulsion of iodide leaving behind Br-rich phases. Hole trapping at I-rich sites in MAPb(Br0.5I0.5)3 is considered to be an important step in inducing halide mobility in photoirradiated films. This Account focuses on halide ion migration in nanocrystals and nanostructured films driven by entropy of mixing in dark and phase segregation under light irradiation.
The optical and electronic properties of metal halide perovskites provide insight into the operation of solar cells as well as their long-term operational stability. Halide mobility in perovskite films is an important factor influencing solar cell performance. One can visualize halide ion migration through halide exchange between two nanocrystal suspensions or between physically paired films of two different metal halide perovskites. The ability to tune band gap by varying halide ratios (Cl:Br or Br:I) allows the synthesis of mixed halide perovskites with tailored absorption and emission across the entire visible spectrum. Interestingly, mixed halide (e.g., MAPb(Br0.5I0.5)3) films undergo phase segregation to form Br-rich and I-rich sites under steady state illumination. Upon halting illumination, segregated phases mix to restore original mixed halide compositions. Introducing multiple cations (Cs, formamidinium) at the A site or alloying with Cl greatly suppresses halide mobilities. Long-term irradiation of MAPb(Br0.5I0.5)3 films also cause expulsion of iodide leaving behind Br-rich phases. Hole trapping at I-rich sites in MAPb(Br0.5I0.5)3 is considered to be an important step in inducing halide mobility in photoirradiated films. This Account focuses on halide ion migration in nanocrystals and nanostructured films driven by entropy of mixing in dark and phase segregation under light irradiation.
545. Electrochemically induced iodine migration in mixed halide perovskites: suppression through chloride insertion Junsang Cho, Jeffrey T. DuBose, Preethi S. Mathew and Prashant V. Kamat Chem. Commun. 2021,57, 235-238
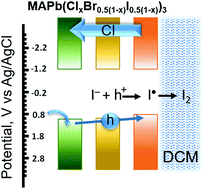 The role of chloride in improving the stability of mixed halide perovskites (MAPbClxBr0.5(1−x)I0.5(1−x))3 is probed using spectroelectrochemistry. The injection of holes into mixed halide perovskite films through applied anodic bias results in the selective migration of iodine with ultimate expulsion into the electrolyte. Increasing the Cl content (x = 0 to 0.1) in the mixed halide perovskite suppresses the iodine mobility and thus decreases the rate of its expulsion into the solution. Implications of iodine mobility induced by hole accumulation and its impact on overall stability is discussed.
The role of chloride in improving the stability of mixed halide perovskites (MAPbClxBr0.5(1−x)I0.5(1−x))3 is probed using spectroelectrochemistry. The injection of holes into mixed halide perovskite films through applied anodic bias results in the selective migration of iodine with ultimate expulsion into the electrolyte. Increasing the Cl content (x = 0 to 0.1) in the mixed halide perovskite suppresses the iodine mobility and thus decreases the rate of its expulsion into the solution. Implications of iodine mobility induced by hole accumulation and its impact on overall stability is discussed.
2020
544. Photoinduced Phase Segregation in Mixed Halide Perovskites: Thermodynamic and Kinetic Aspects of Cl–Br Segregation Junsang Cho & Prashant V. Kamat Advanced Optical Materials 2020, 2001440.
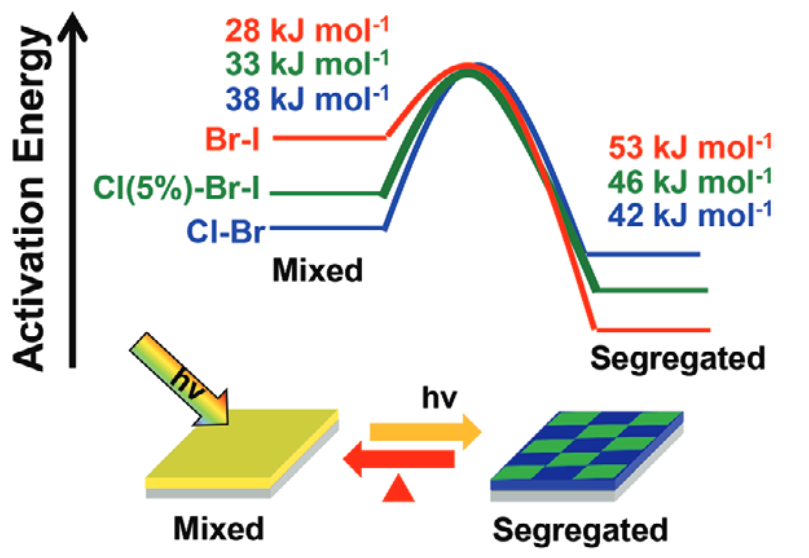 Suppression of halide ion mobility remains a key issue in defining the device performances and stability of perovskite solar cells. The halide ion migration is facilitated by the lattice-distortion-mediated halide vacancy and hence dictated by the polarizability (or rigidity) of the halide sublattice and framework. Photoinduced halide ion segregation and dark recovery of MAPb(Cl0.5Br0.5)3 films are now probed. The temperature dependence of the rate constants of segregation and dark remixing allow to determine the activation energy barriers (Ea) for photosegregation (38 kJ mol−1) and dark recovery (42 kJ mol−1). A comparison of the segregation activation energy (Ea) and excitation intensity threshold between MAPb(Cl0.5Br0.5)3 and MAPb(Br0.5I0.5)3 films reflects that the presence of Cl stabilizes mixed halide composition. The thermodynamic stabilization is attributed to the formation of more rigid [PbX6]4− frameworks with an increased barrier for halide ion migration. The thermodynamic rationale behind the intriguing halide ion mobility offers a fundamental insight into the role of Cl and design principle of perovskite solar cells with improved efficiency and long-term stability.
Suppression of halide ion mobility remains a key issue in defining the device performances and stability of perovskite solar cells. The halide ion migration is facilitated by the lattice-distortion-mediated halide vacancy and hence dictated by the polarizability (or rigidity) of the halide sublattice and framework. Photoinduced halide ion segregation and dark recovery of MAPb(Cl0.5Br0.5)3 films are now probed. The temperature dependence of the rate constants of segregation and dark remixing allow to determine the activation energy barriers (Ea) for photosegregation (38 kJ mol−1) and dark recovery (42 kJ mol−1). A comparison of the segregation activation energy (Ea) and excitation intensity threshold between MAPb(Cl0.5Br0.5)3 and MAPb(Br0.5I0.5)3 films reflects that the presence of Cl stabilizes mixed halide composition. The thermodynamic stabilization is attributed to the formation of more rigid [PbX6]4− frameworks with an increased barrier for halide ion migration. The thermodynamic rationale behind the intriguing halide ion mobility offers a fundamental insight into the role of Cl and design principle of perovskite solar cells with improved efficiency and long-term stability.
543. Optimization of the electron transport layer in quantum dot light-emitting devices Gary Zaiats, Shingo Ikeda & Prashant V. Kamat NPG Asia Materials 12, Article number: 57 (2020).
 Quantum dot light-emitting devices have emerged as an important technology for display applications. Their emission is a result of recombination between positive and negative charge carriers that are transported through the hole and electron conductive layers, respectively. The selection of electron or hole transport materials in these devices not only demands the alignment of energy levels between the layers but also balances the flow of electrons and holes toward the recombination sites. In this work, we examine a method for device optimization through control of the charge carrier kinetics. We employ impedance spectroscopy to examine the mobility of charge carriers through each of the layers. The derived mobility values provide a path to estimate the transition time of each charge carrier toward the emitting layer. We suggest that an optimal device structure can be obtained when the transition times of both charge carriers toward the active layer are similar. Finally, we examine our hypothesis by focusing on thickness optimization of the electron transport layer.
Quantum dot light-emitting devices have emerged as an important technology for display applications. Their emission is a result of recombination between positive and negative charge carriers that are transported through the hole and electron conductive layers, respectively. The selection of electron or hole transport materials in these devices not only demands the alignment of energy levels between the layers but also balances the flow of electrons and holes toward the recombination sites. In this work, we examine a method for device optimization through control of the charge carrier kinetics. We employ impedance spectroscopy to examine the mobility of charge carriers through each of the layers. The derived mobility values provide a path to estimate the transition time of each charge carrier toward the emitting layer. We suggest that an optimal device structure can be obtained when the transition times of both charge carriers toward the active layer are similar. Finally, we examine our hypothesis by focusing on thickness optimization of the electron transport layer.
542. How Chloride Suppresses Photoinduced Phase Segregation in Mixed Halide Perovskites Junsang Cho and Prashant V. Kamat Chem. Mater. 2020, 32, 14, 6206–6212.
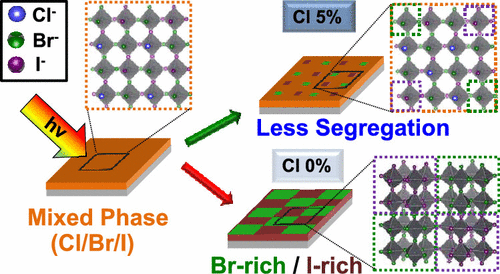 Halide ion mobility in metal halide perovskites plays an important role in dictating the overall device performance and long-term stability of perovskite solar cells. Alloying with chloride (Cl), which is known to stabilize the perovskite solar cells, has now been found to suppress the photoinduced halide ion segregation in mixed halide (Br/I) perovskites. By varying the chloride concentration of 1–10% (as part of the halide composition), we have probed both photoinduced segregation and dark recovery kinetics at different temperatures. When we increased the concentration of Cl from 0 to 5%, we observed a decrease in the rate constant of segregation by a factor of ∼5 and a decrease in the fraction of halide segregation from 45 to 20%. The activation energy for photoinduced halide segregation increases (∼4 kJ/mol) upon the introduction of chloride into the mixed halide film, reflecting an increased energetic barrier for halide ion migration.
Halide ion mobility in metal halide perovskites plays an important role in dictating the overall device performance and long-term stability of perovskite solar cells. Alloying with chloride (Cl), which is known to stabilize the perovskite solar cells, has now been found to suppress the photoinduced halide ion segregation in mixed halide (Br/I) perovskites. By varying the chloride concentration of 1–10% (as part of the halide composition), we have probed both photoinduced segregation and dark recovery kinetics at different temperatures. When we increased the concentration of Cl from 0 to 5%, we observed a decrease in the rate constant of segregation by a factor of ∼5 and a decrease in the fraction of halide segregation from 45 to 20%. The activation energy for photoinduced halide segregation increases (∼4 kJ/mol) upon the introduction of chloride into the mixed halide film, reflecting an increased energetic barrier for halide ion migration.
541. Surface Chemistry Matters. How Ligands Influence Excited State Interactions between CsPbBr3 and Methyl Viologen Jeffrey T. DuBose and Prashant V. Kamat J. Phys. Chem. C 2020, 124, 24, 12990–12998.
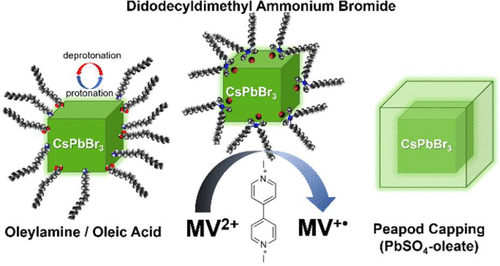 The photocatalytic properties of cesium lead bromide (CsPbBr3) perovskite nanocrystals make them attractive for designing light harvesting assemblies. Often ignored, the surface chemistry can dictate the excited state interactions of these semiconductor nanocrystals with charge-shuttling redox molecules. We have now explored the impact of CsPbBr3 nanocrystal surface modification on the excited state interactions with methyl viologen (MV2+) for three different ligand environments: prototypical oleic acid/oleylamine (OA/OAm) ligands, PbSO4-oleate capping, and didodecyldimethylammonium bromide (DDAB) ligands. Native OA/OAm ligands and PbSO4-oleate capping exhibit the strongest complexation with MV2+, whereas the bulky DDAB ligand environment shows an order of magnitude weaker complexation. The electron transfer rate constants as measured from transient absorption spectroscopy vary in the range of 1.2–3.6 × 1011 s–1 for different ligand environments. For DDAB-CsPbBr3 NCs, the efficiency of electron transfer (Φet) is 73%. Despite a protective capping layer, PbSO4-oleate capped CsPbBr3 maintains a redox-active surface which is viable for photocatalytic applications. These results highlight the impact of surface chemistry on excited state interactions of CsPbBr3 NCs and photocatalytic applications.
The photocatalytic properties of cesium lead bromide (CsPbBr3) perovskite nanocrystals make them attractive for designing light harvesting assemblies. Often ignored, the surface chemistry can dictate the excited state interactions of these semiconductor nanocrystals with charge-shuttling redox molecules. We have now explored the impact of CsPbBr3 nanocrystal surface modification on the excited state interactions with methyl viologen (MV2+) for three different ligand environments: prototypical oleic acid/oleylamine (OA/OAm) ligands, PbSO4-oleate capping, and didodecyldimethylammonium bromide (DDAB) ligands. Native OA/OAm ligands and PbSO4-oleate capping exhibit the strongest complexation with MV2+, whereas the bulky DDAB ligand environment shows an order of magnitude weaker complexation. The electron transfer rate constants as measured from transient absorption spectroscopy vary in the range of 1.2–3.6 × 1011 s–1 for different ligand environments. For DDAB-CsPbBr3 NCs, the efficiency of electron transfer (Φet) is 73%. Despite a protective capping layer, PbSO4-oleate capped CsPbBr3 maintains a redox-active surface which is viable for photocatalytic applications. These results highlight the impact of surface chemistry on excited state interactions of CsPbBr3 NCs and photocatalytic applications.
540. Iodine (I) Expulsion at Photoirradiated Mixed Halide Perovskite Interface. Should I Stay or Should I Go? Preethi Susan Mathew, Gergely F. Samu, Csaba Janáky, and Prashant V. Kamat ACS Energy Lett. 2020, 5, 6, 1872–1880.
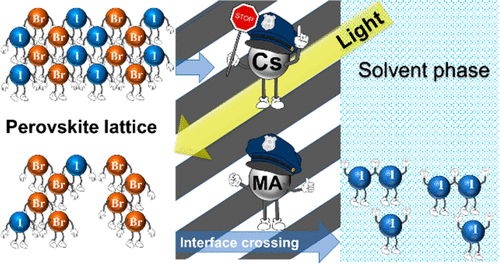 Visible light irradiation of a mixed halide perovskite film in contact with a solvent (dichloromethane, DCM) in which the film otherwise is stable leads to selective expulsion of iodide (I) from the film with a concurrent shift in the band edge to lower wavelengths. We have now employed mixed halide perovskites to uncover the influence of A-site cation [methylammonium (MA) and cesium (Cs)] on the mobility of iodide ions under photoirradiation. In the absence of solvent contact, the mixed halide perovskite films undergo photoinduced segregation with a rate constant that decreases with increasing Cs content. Interestingly, the iodide expulsion rate in DCM is strongly dependent on the rate of photoinduced segregation. At Cs atomic concentrations greater than 50%, the films become stable as the iodide expulsion is largely suppressed. The role of the A-site cation in dictating the mobility of halide ions is discussed.
Visible light irradiation of a mixed halide perovskite film in contact with a solvent (dichloromethane, DCM) in which the film otherwise is stable leads to selective expulsion of iodide (I) from the film with a concurrent shift in the band edge to lower wavelengths. We have now employed mixed halide perovskites to uncover the influence of A-site cation [methylammonium (MA) and cesium (Cs)] on the mobility of iodide ions under photoirradiation. In the absence of solvent contact, the mixed halide perovskite films undergo photoinduced segregation with a rate constant that decreases with increasing Cs content. Interestingly, the iodide expulsion rate in DCM is strongly dependent on the rate of photoinduced segregation. At Cs atomic concentrations greater than 50%, the films become stable as the iodide expulsion is largely suppressed. The role of the A-site cation in dictating the mobility of halide ions is discussed.
539. Suppressed Halide Ion Migration in 2D Lead Halide Perovskites Junsang Cho, Jeffrey T. DuBose, An Ngoc Thien Le, and Prashant V. Kamat ACS Materials Lett. 2020, 2, 6, 565–570.
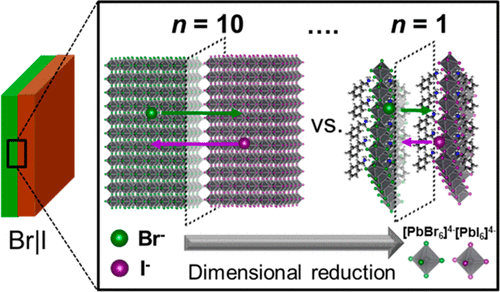 Two-dimensional (2D) lead halide perovskites represent an emerging class of materials given their tunable optoelectronic properties and long-term stability in perovskite solar cells. In order to assess the halide ion mobility, we have tracked the changes in the bromide and iodide composition in physically paired 2D lead halide perovskite films of different layer numbers (n = 10–1). These low-dimensional perovskites suppressed halide ion migration as a result of their intercalated spacer ligands and their strong van der Waals interactions. The rate constants for halide exchange of low dimensionality perovskites follow the Arrhenius relationship with thermal activation energy ranging from 58 kJ/mol (n = 10) to 72 kJ/mol (n = 1). The suppression of halide ion mobility (and diffusion coefficient) with modulating perovskite layer number (n) provides further insight into the role of 2D perovskites in improving the performance of photovoltaic devices.
Two-dimensional (2D) lead halide perovskites represent an emerging class of materials given their tunable optoelectronic properties and long-term stability in perovskite solar cells. In order to assess the halide ion mobility, we have tracked the changes in the bromide and iodide composition in physically paired 2D lead halide perovskite films of different layer numbers (n = 10–1). These low-dimensional perovskites suppressed halide ion migration as a result of their intercalated spacer ligands and their strong van der Waals interactions. The rate constants for halide exchange of low dimensionality perovskites follow the Arrhenius relationship with thermal activation energy ranging from 58 kJ/mol (n = 10) to 72 kJ/mol (n = 1). The suppression of halide ion mobility (and diffusion coefficient) with modulating perovskite layer number (n) provides further insight into the role of 2D perovskites in improving the performance of photovoltaic devices.
538. Charge Carrier Recombination Dynamics of Two-Dimensional Lead Halide Perovskites Junsang Cho, Jeffrey T. DuBose and Prashant V. Kamat J. Phys. Chem. Lett. 2020, 11, 7, 2570-2576
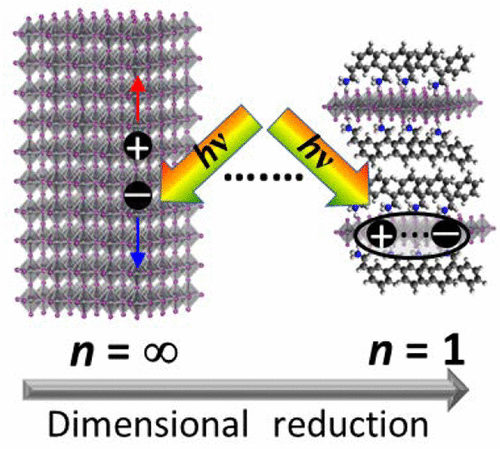 Two-dimensional (2D) lead halide perovskites with better chemical stability and tunable dimensionality offer new opportunities to design optoelectronic devices. We have probed the transient absorption behavior of 2D lead halide (bromide and iodide) perovskites of different dimensionality, prepared by varying the ratio of methylammonium:phenylethylammonium cation. With decreasing dimensionality (n = ∞ → 1), we observe a blue shift in transient absorption bleach in agreement with the trend observed with the shift in the excitonic peak. The lifetime of the charge carriers decreased with decreasing layer thickness. The dependence of charge carrier lifetime on the 2D layers as well as the halide ion composition shows the dominance of excitonic binding energy on the charge carrier recombination in 2D perovskites. The excited-state behavior of 2D perovskites discussed in this study shows the need to modulate the layer dimensionality to obtain desired optoelectronic properties.
Two-dimensional (2D) lead halide perovskites with better chemical stability and tunable dimensionality offer new opportunities to design optoelectronic devices. We have probed the transient absorption behavior of 2D lead halide (bromide and iodide) perovskites of different dimensionality, prepared by varying the ratio of methylammonium:phenylethylammonium cation. With decreasing dimensionality (n = ∞ → 1), we observe a blue shift in transient absorption bleach in agreement with the trend observed with the shift in the excitonic peak. The lifetime of the charge carriers decreased with decreasing layer thickness. The dependence of charge carrier lifetime on the 2D layers as well as the halide ion composition shows the dominance of excitonic binding energy on the charge carrier recombination in 2D perovskites. The excited-state behavior of 2D perovskites discussed in this study shows the need to modulate the layer dimensionality to obtain desired optoelectronic properties.
537. Photoinduced Anion Segregation in Mixed Halide Perovskites (Review) Michael C. Brennan, Anthony Ruth, Prashant V. Kamat, and Masaru Kuno Trends in Chemistry Volume 2, Issue 4, April 2020, Pages 282-301
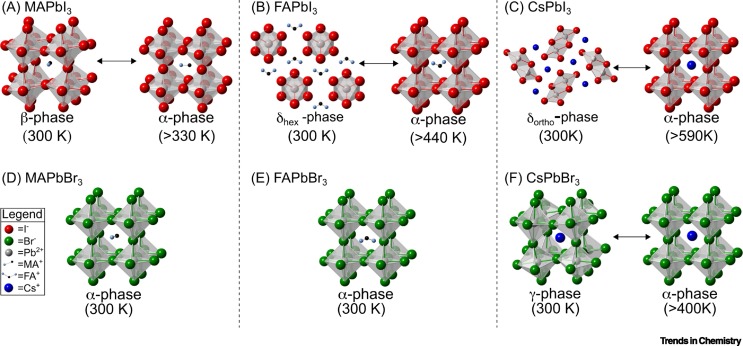 Alloyed lead halide perovskites have taken a dominant role in the quest for third-generation solar cells. This is due to optimal light-harvesting properties, which can be tuned across the visible spectrum by mixing halide (X = Cl–, Br–, and I–) anions and A+ cations (A+ = FA+, MA+, and Cs+). Durability issues related to ion movement within the perovskite lattice, however, impede large-scale commercialization. Uniformly mixed halide perovskites [e.g., APb(I1–xBrx)3] reversibly segregate into narrow bandgap I-rich and wide bandgap Br-rich domains during continuous visible illumination. Subsequent I-rich domains reduce local open circuit voltages and decrease mixed halide perovskite solar cell power conversion efficiencies. In this review, we assess the known effects of halide segregation on the structural and optical properties of mixed halide materials, discuss ongoing research to suppress the phenomenon, and provide a mechanistic overview of its underlying origins.
Alloyed lead halide perovskites have taken a dominant role in the quest for third-generation solar cells. This is due to optimal light-harvesting properties, which can be tuned across the visible spectrum by mixing halide (X = Cl–, Br–, and I–) anions and A+ cations (A+ = FA+, MA+, and Cs+). Durability issues related to ion movement within the perovskite lattice, however, impede large-scale commercialization. Uniformly mixed halide perovskites [e.g., APb(I1–xBrx)3] reversibly segregate into narrow bandgap I-rich and wide bandgap Br-rich domains during continuous visible illumination. Subsequent I-rich domains reduce local open circuit voltages and decrease mixed halide perovskite solar cell power conversion efficiencies. In this review, we assess the known effects of halide segregation on the structural and optical properties of mixed halide materials, discuss ongoing research to suppress the phenomenon, and provide a mechanistic overview of its underlying origins.
536. TiO2-Assisted Halide Ion Segregation in Mixed Halide Perovskite Films Jeffrey T. DuBose and Prashant V. Kamat J. Am. Chem. Soc. 2020, 142, 11, 5362–5370.
 In metal halide perovskite solar cells, electron transport layers (ETLs) such as TiO2 dictate the overall photovoltaic performance. However, the same electron capture property of ETL indirectly impacts halide ion mobility as evident from the TiO2-assisted halide ion segregation in mixed halide perovskite (MHP) films under pulsed laser excitation (387 nm, 500 Hz). This segregation is only observed when deposited on an ETL such as TiO2 but not on insulating ZrO2 substrate. Injection of electrons from excited MHP into the ETL (ket = 1011 s–1) followed by scavenging of electrons by O2 causes hole accumulation in the MHP film. Localization of holes on the iodide site in the MHP induces instability causing iodide from the lattice to move away toward grain boundaries. Suppression of segregation occurs when holes are extracted by a hole transport layer (spiro-OMeTAD) deposited on the MHP, thus avoiding hole build-up. These results provide further insight into the role of holes in the phase segregation of MHPs and hole mobility in perovskite solar cells.
In metal halide perovskite solar cells, electron transport layers (ETLs) such as TiO2 dictate the overall photovoltaic performance. However, the same electron capture property of ETL indirectly impacts halide ion mobility as evident from the TiO2-assisted halide ion segregation in mixed halide perovskite (MHP) films under pulsed laser excitation (387 nm, 500 Hz). This segregation is only observed when deposited on an ETL such as TiO2 but not on insulating ZrO2 substrate. Injection of electrons from excited MHP into the ETL (ket = 1011 s–1) followed by scavenging of electrons by O2 causes hole accumulation in the MHP film. Localization of holes on the iodide site in the MHP induces instability causing iodide from the lattice to move away toward grain boundaries. Suppression of segregation occurs when holes are extracted by a hole transport layer (spiro-OMeTAD) deposited on the MHP, thus avoiding hole build-up. These results provide further insight into the role of holes in the phase segregation of MHPs and hole mobility in perovskite solar cells.
2019
535. Perovskite Photocatalysis. Methyl Viologen Induces Unusually Long-Lived Charge Carrier Separation in CsPbBr3 Nanocrystals Steven M. Kobosko, Jeffrey T. DuBose and Prashant V. Kamat ACS Energy Lett. 2020, 5, 1, 221–223.
 The strong binding between CsPbBr3 nanocrystals and methyl viologen induces a long-lived charge-separated state following band gap excitation with important implications in photocatalytic processes. The unusually long-lived bleaching of the CsPbBr3 excitonic peak in this case arises from the creation of a dipole with the hole residing in CsPbBr3 and the electron in the surface-bound methyl viologen moiety.
The strong binding between CsPbBr3 nanocrystals and methyl viologen induces a long-lived charge-separated state following band gap excitation with important implications in photocatalytic processes. The unusually long-lived bleaching of the CsPbBr3 excitonic peak in this case arises from the creation of a dipole with the hole residing in CsPbBr3 and the electron in the surface-bound methyl viologen moiety.
534. Charge Injection from Excited Cs2AgBiBr6 Quantum Dots into Semiconductor Oxides Junsang Cho, Jeffrey T. DuBose and Prashant V. Kamat Chem. Mater. 2020, 32, 1, 510–517.
 Lead-free double perovskites such as Cs2AgBiBr6 are gaining attention because of their environmental friendliness compared to the lead halide perovskites. In order to establish their photoactivity, we have probed the excited-state behavior of Cs2AgBiBr6 nanocrystals and charge injection from their excited state into different metal oxides (TiO2, ZnO). The electron-transfer rate constants determined from ultrafast transient absorption spectroscopy were in the range of 1.2–5.2 × 1010 s–1. Under steady-state photolysis (ambient conditions), the electrons injected into TiO2 are scavenged by atmospheric oxygen, leaving behind holes which accumulate within the quantum dots (QDs). These accumulated holes further induce oxidation of QDs, resulting in the overall photodegradation of perovskite films. Annealed films of Cs2AgBiBr6 nanocrystals, when employed as an active layer in solar cells, delivered photocurrent under visible-light excitation.
Lead-free double perovskites such as Cs2AgBiBr6 are gaining attention because of their environmental friendliness compared to the lead halide perovskites. In order to establish their photoactivity, we have probed the excited-state behavior of Cs2AgBiBr6 nanocrystals and charge injection from their excited state into different metal oxides (TiO2, ZnO). The electron-transfer rate constants determined from ultrafast transient absorption spectroscopy were in the range of 1.2–5.2 × 1010 s–1. Under steady-state photolysis (ambient conditions), the electrons injected into TiO2 are scavenged by atmospheric oxygen, leaving behind holes which accumulate within the quantum dots (QDs). These accumulated holes further induce oxidation of QDs, resulting in the overall photodegradation of perovskite films. Annealed films of Cs2AgBiBr6 nanocrystals, when employed as an active layer in solar cells, delivered photocurrent under visible-light excitation.
532. How Interplay between Photo and Thermal Activation Dictates Halide Ion Segregation in Mixed Halide Perovskites Tor Elmelund, Brian Seger, Masaru Kuno and Prashant V. Kamat ACS Energy Lett. 2020, 5, 1, 56–63.
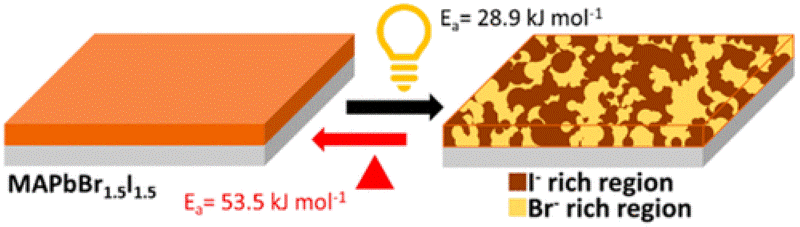 The halide ion mobility in mixed halide perovskite exhibits two opposite trends in response to photo and thermal activation. While halides prefer to remain as Br-rich and I-rich domains under steady-state light irradiation of MAPbBr1.5I1.5 films, they prefer to remain in their stable mixed composition when kept in the dark. The activation energies as determined from the temperature-dependent rate constants are Ea,forward = 28.9 kJ mol–1 for photoinduced segregation and Ea,reverse = 53.5 kJ mol–1 for remixing of halides in the dark, respectively. The energy input from photoexcitation assists overcoming the dark (thermally activated) mixing to induce Br-rich and I-rich domains. This segregated state is maintained as long as the mixed halide film is irradiated continuously with visible light. The excitation intensity threshold above which segregation occurs follows a linear temperature dependence, such that phase separation occurs above Iexc = 30 μW/cm2 white light at 23 °C. The threshold at 90 °C becomes higher with a minimum intensity requirement of 100 μW/cm2 to induce segregation. The thermodynamic rationale behind this unusual halide mobility under photo and thermal excitation discussed here can aid in understanding the stability issues of perovskite solar cells.
The halide ion mobility in mixed halide perovskite exhibits two opposite trends in response to photo and thermal activation. While halides prefer to remain as Br-rich and I-rich domains under steady-state light irradiation of MAPbBr1.5I1.5 films, they prefer to remain in their stable mixed composition when kept in the dark. The activation energies as determined from the temperature-dependent rate constants are Ea,forward = 28.9 kJ mol–1 for photoinduced segregation and Ea,reverse = 53.5 kJ mol–1 for remixing of halides in the dark, respectively. The energy input from photoexcitation assists overcoming the dark (thermally activated) mixing to induce Br-rich and I-rich domains. This segregated state is maintained as long as the mixed halide film is irradiated continuously with visible light. The excitation intensity threshold above which segregation occurs follows a linear temperature dependence, such that phase separation occurs above Iexc = 30 μW/cm2 white light at 23 °C. The threshold at 90 °C becomes higher with a minimum intensity requirement of 100 μW/cm2 to induce segregation. The thermodynamic rationale behind this unusual halide mobility under photo and thermal excitation discussed here can aid in understanding the stability issues of perovskite solar cells.
531. Temperature-driven anion migration in gradient halide perovskites Rebecca A. Scheidt and Prashant V. Kamat J. Chem. Phys. 151, 134703 (2019)
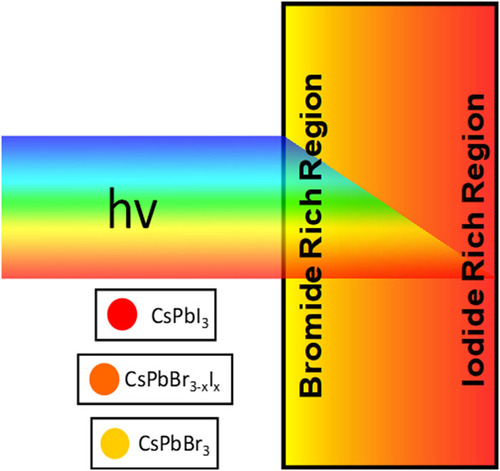 Cesium lead halide perovskite films with a systematic change in the halide composition of CsPbBr3−xIx, in which iodide concentration varies from x = 0 to x = 3, provide a built-in gradient band structure. Such a gradient structure allows for the integrated capture of visible photons and directs them to the energetically low-lying iodide rich region. Annealing gradient halide perovskite films at temperatures ranging from 50 °C to 90 °C causes the films to homogenize into mixed halide perovskites. The movement of halide ions during the homogenization process was elucidated using UV-Visible absorbance and X-ray photoelectron spectroscopy. The halide ion movement in CsPbBr3−xIx gradient films was tracked via absorbance changes in the visible region of the spectrum that enabled us to measure the temperature dependent rate constant and energy of activation (74.5 kJ/mol) of halide ion homogenization. Excited state processes of both gradient and homogenized films probed through transient absorption spectroscopy showed the direct flow of charge carriers and charge recombination in both films.
Cesium lead halide perovskite films with a systematic change in the halide composition of CsPbBr3−xIx, in which iodide concentration varies from x = 0 to x = 3, provide a built-in gradient band structure. Such a gradient structure allows for the integrated capture of visible photons and directs them to the energetically low-lying iodide rich region. Annealing gradient halide perovskite films at temperatures ranging from 50 °C to 90 °C causes the films to homogenize into mixed halide perovskites. The movement of halide ions during the homogenization process was elucidated using UV-Visible absorbance and X-ray photoelectron spectroscopy. The halide ion movement in CsPbBr3−xIx gradient films was tracked via absorbance changes in the visible region of the spectrum that enabled us to measure the temperature dependent rate constant and energy of activation (74.5 kJ/mol) of halide ion homogenization. Excited state processes of both gradient and homogenized films probed through transient absorption spectroscopy showed the direct flow of charge carriers and charge recombination in both films.
530. Probing Perovskite Photocatalysis. Interfacial Electron Transfer between CsPbBr3 and Ferrocene Redox Couple Jeffrey T. DuBose and Prashant V. Kamat J. Phys. Chem. Lett. 2019, 10, 20, 6074-6080
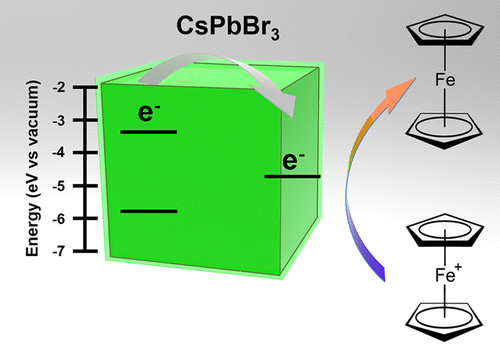 Interfacial charge transfer between a semiconductor nanocrystal and a molecular relay is an important step in nanomaterial photocatalysis. The ferrocene redox couple (Fc+/Fc0, E0 = −4.9 eV vs vacuum) has now been used as a model redox relay system to investigate photocatalytic properties of CsPbBr3 perovskite nanocrystals. The photocatalytic reduction of ferrocenium (Fc+) to ferrocene (Fc0) with CsPbBr3 nanocrystals was dictated by the surface interactions. Whereas a rapid quenching and subsequent recovery of CsPbBr3 emission is seen at low Fc+ concentrations, the quenched emission was sustained at higher Fc+ concentrations. The photoinduced interfacial electron transfer between CsPbBr3 and ferrocenium (Fc+) studied using transient absorption spectroscopy occurred with a rate constant of 1.64 × 1010 s–1. Better understanding of interfacial processes using redox probes can lead to the improvement in photocatalytic performance of perovskite nanocrystals.
Interfacial charge transfer between a semiconductor nanocrystal and a molecular relay is an important step in nanomaterial photocatalysis. The ferrocene redox couple (Fc+/Fc0, E0 = −4.9 eV vs vacuum) has now been used as a model redox relay system to investigate photocatalytic properties of CsPbBr3 perovskite nanocrystals. The photocatalytic reduction of ferrocenium (Fc+) to ferrocene (Fc0) with CsPbBr3 nanocrystals was dictated by the surface interactions. Whereas a rapid quenching and subsequent recovery of CsPbBr3 emission is seen at low Fc+ concentrations, the quenched emission was sustained at higher Fc+ concentrations. The photoinduced interfacial electron transfer between CsPbBr3 and ferrocenium (Fc+) studied using transient absorption spectroscopy occurred with a rate constant of 1.64 × 1010 s–1. Better understanding of interfacial processes using redox probes can lead to the improvement in photocatalytic performance of perovskite nanocrystals.
529. Bidirectional Halide Ion Exchange in Paired Lead Halide Perovskite Films with Thermal Activation Tor Elmelund, Rebecca A. Scheidt, Brian Seger, Prashant V. Kamat ACS Energy Lett. 2019, 4, XXX, 1961-1969
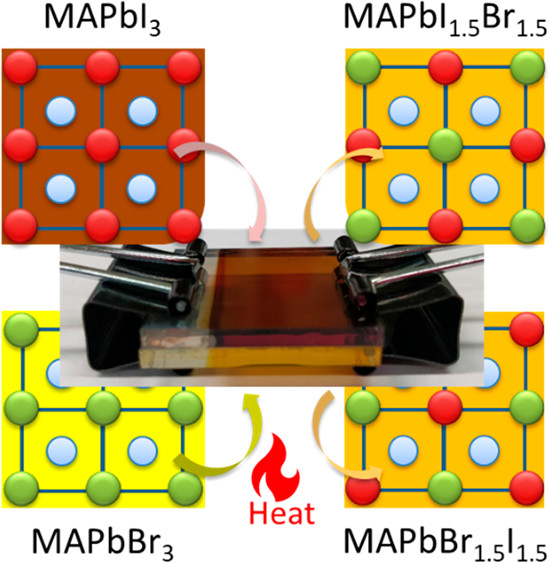 MAPbBr3 and MAPbI3 films cast onto glass slides and physically paired together undergo halide exchange to form mixed halide films. The change in halide composition in these two ∼130 nm thick films occurs concurrently with Br– diffusing toward the MAPbI3 film and I– diffusing toward the MAPbBr3 film. The diffusion of these halide species, which is tracked through changes in the absorption, offers a direct measurement of thermally activated halide diffusion in perovskite films. The increase in the rate constant of halide diffusion with increasing temperature (from 8.3 × 10–6 s–1 at 23 °C to 3.7 × 10–4 s–1 at 140 °C) follows an Arrhenius relationship with activation energy of 51 kJ/mol. The thermally activated halide exchange shows the challenges of employing layers of different metal halide perovskites in stable tandem solar cells.
MAPbBr3 and MAPbI3 films cast onto glass slides and physically paired together undergo halide exchange to form mixed halide films. The change in halide composition in these two ∼130 nm thick films occurs concurrently with Br– diffusing toward the MAPbI3 film and I– diffusing toward the MAPbBr3 film. The diffusion of these halide species, which is tracked through changes in the absorption, offers a direct measurement of thermally activated halide diffusion in perovskite films. The increase in the rate constant of halide diffusion with increasing temperature (from 8.3 × 10–6 s–1 at 23 °C to 3.7 × 10–4 s–1 at 140 °C) follows an Arrhenius relationship with activation energy of 51 kJ/mol. The thermally activated halide exchange shows the challenges of employing layers of different metal halide perovskites in stable tandem solar cells.
528. Electrochemical Hole Injection Selectively Expels Iodide from Mixed Halide Perovskite Films Gergely F. Samu, Ádám Balog, Filippo De Angelis, Daniele Meggiolaro, Prashant V. Kamat, and Csaba Janáky J. Am. Chem. Soc. 2019, 141, 27, 10812-10820
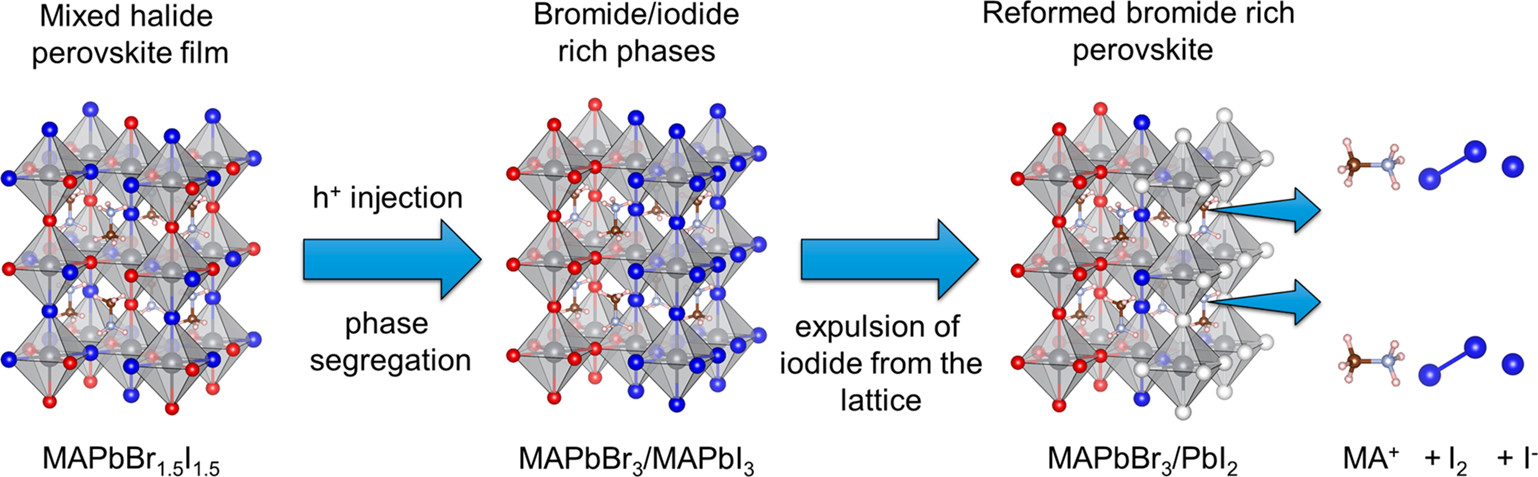 Halide ion mobility in metal halide perovskites remains an intriguing phenomenon, influencing their optical and photovoltaic properties. Selective injection of holes through electrochemical anodic bias has allowed us to probe the effect of hole trapping at iodide (0.9 V) and bromide (1.15 V) in mixed halide perovskite (CH3NH3PbBr1.5I1.5) films. Upon trapping holes at the iodide site, the iodide gradually gets expelled from the mixed halide film (as iodine and/or triiodide ion), leaving behind re-formed CH3NH3PbBr3 domains. The weakening of the Pb–I bond following the hole trapping (oxidation of the iodide site) and its expulsion from the lattice in the form of iodine provided further insight into the photoinduced segregation of halide ions in mixed halide perovskite films. Transient absorption spectroscopy revealed that the iodide expulsion process leaves a defect-rich perovskite lattice behind as charge carrier recombination in the re-formed lattice is greatly accelerated. The selective mobility of iodide species provides insight into the photoinduced phase segregation and its implication in the stable operation of perovskite solar cells.
Halide ion mobility in metal halide perovskites remains an intriguing phenomenon, influencing their optical and photovoltaic properties. Selective injection of holes through electrochemical anodic bias has allowed us to probe the effect of hole trapping at iodide (0.9 V) and bromide (1.15 V) in mixed halide perovskite (CH3NH3PbBr1.5I1.5) films. Upon trapping holes at the iodide site, the iodide gradually gets expelled from the mixed halide film (as iodine and/or triiodide ion), leaving behind re-formed CH3NH3PbBr3 domains. The weakening of the Pb–I bond following the hole trapping (oxidation of the iodide site) and its expulsion from the lattice in the form of iodine provided further insight into the photoinduced segregation of halide ions in mixed halide perovskite films. Transient absorption spectroscopy revealed that the iodide expulsion process leaves a defect-rich perovskite lattice behind as charge carrier recombination in the re-formed lattice is greatly accelerated. The selective mobility of iodide species provides insight into the photoinduced phase segregation and its implication in the stable operation of perovskite solar cells.
527. Tracking Transformative Transitions: From CsPbBr3 Nanocrystals to Bulk Perovskite Films Rebecca A. Scheidt, Corey Atwell, and Prashant V. Kamat ACS Materials Letters 2019
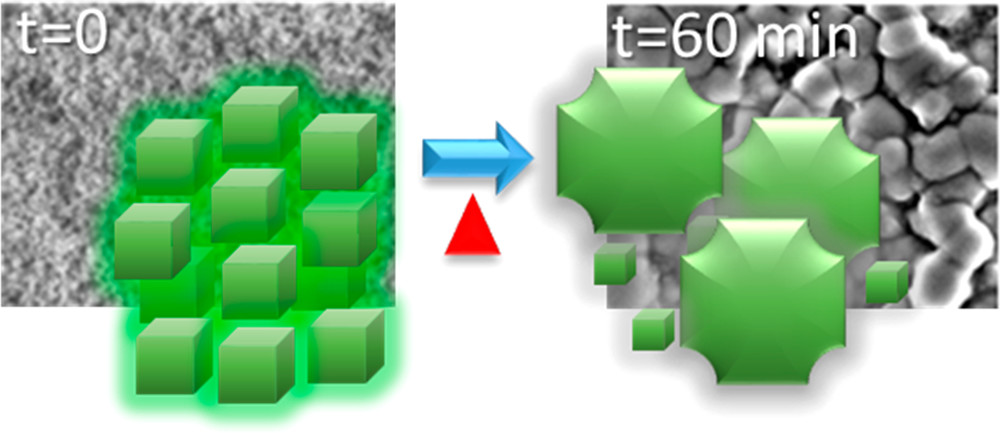 The control of grain size and surface properties is an important parameter in controlling the optoelectronic and photovoltaic properties of metal halide perovskites. When CsPbBr3 nanocrystal (∼10 nm in diameter) films were annealed at 100–125 °C, they grow in size to produce ∼400 nm diameter crystallites while transforming into bulk perovskite films. Characteristic changes in the optical properties were noted when such transformation occurred from nanocrystals into bulk. By tracking absorbance and emission spectra and morphological changes of CsPbBr3 films at different annealing times and temperature, we were able to establish the mechanism of particle growth. The presence of nanocrystals and larger crystals during the intermediate annealing steps and narrowing size distribution confirmed the Ostwald ripening mechanism for the crystal growth. The energy of activation of crystal growth as determined from the temperature dependent optical properties was estimated to be 75 kcal/mol.
The control of grain size and surface properties is an important parameter in controlling the optoelectronic and photovoltaic properties of metal halide perovskites. When CsPbBr3 nanocrystal (∼10 nm in diameter) films were annealed at 100–125 °C, they grow in size to produce ∼400 nm diameter crystallites while transforming into bulk perovskite films. Characteristic changes in the optical properties were noted when such transformation occurred from nanocrystals into bulk. By tracking absorbance and emission spectra and morphological changes of CsPbBr3 films at different annealing times and temperature, we were able to establish the mechanism of particle growth. The presence of nanocrystals and larger crystals during the intermediate annealing steps and narrowing size distribution confirmed the Ostwald ripening mechanism for the crystal growth. The energy of activation of crystal growth as determined from the temperature dependent optical properties was estimated to be 75 kcal/mol.
526. Ag(I)-Thiolate-Protected Silver Nanoclusters for Solar Cells: Electrochemical and Spectroscopic Look into the Photoelectrode/Electrolyte Interface Muhammad A. Abbas, Seog Joon Yoon, Hahkjoon Kim, Junghyun Lee, Prashant V. Kamat, and Jin Ho Bang ACS Appl. Mater. Interfaces 2019
 Intrinsic low stability and short excited lifetimes associated with Ag nanoclusters (NCs) are major hurdles that have prevented the full utilization of the many advantages of Ag NCs over their longtime contender, Au NCs, in light energy conversion systems. In this report, we diagnosed the problems of conventional thiolated Ag NCs used for solar cell applications and developed a new synthesis route to form aggregation-induced emission (AIE)-type Ag NCs that can significantly overcome these limitations. A series of Ag(0)/Ag(I)-thiolate core/shell-structured NCs with different core sizes were explored for photoelectrodes, and the nature of the two important interfacial events occurring in Ag NC-sensitized solar cells (photoinduced electron transfer and charge recombination) were unveiled by in-depth spectroscopic and electrochemical analyses. This work reveals that the subtle interplay between the light absorbing capability, charge separation dynamics, and charge recombination kinetics in the photoelectrode dictates the solar cell performance. In addition, we demonstrate significant improvement in the photocurrent stability and light conversion efficiency that have not been achieved previously. Our comprehensive understanding of the critical parameters that limit the light conversion efficiency lays a foundation on which new principles for designing Ag NCs for efficient light energy conversion can be built.
Intrinsic low stability and short excited lifetimes associated with Ag nanoclusters (NCs) are major hurdles that have prevented the full utilization of the many advantages of Ag NCs over their longtime contender, Au NCs, in light energy conversion systems. In this report, we diagnosed the problems of conventional thiolated Ag NCs used for solar cell applications and developed a new synthesis route to form aggregation-induced emission (AIE)-type Ag NCs that can significantly overcome these limitations. A series of Ag(0)/Ag(I)-thiolate core/shell-structured NCs with different core sizes were explored for photoelectrodes, and the nature of the two important interfacial events occurring in Ag NC-sensitized solar cells (photoinduced electron transfer and charge recombination) were unveiled by in-depth spectroscopic and electrochemical analyses. This work reveals that the subtle interplay between the light absorbing capability, charge separation dynamics, and charge recombination kinetics in the photoelectrode dictates the solar cell performance. In addition, we demonstrate significant improvement in the photocurrent stability and light conversion efficiency that have not been achieved previously. Our comprehensive understanding of the critical parameters that limit the light conversion efficiency lays a foundation on which new principles for designing Ag NCs for efficient light energy conversion can be built.
525. Tuning the Excited-State Dynamics of CuI Films with Electrochemical Bias Gergely F. Samu, Rebecca A. Scheidt, Ádám Balog, Csaba Janáky, and Prashant V. Kamat ACS Energy Lett. 2019, 4, pp 702–708
 Owing to its high hole conductivity and ease of preparation, CuI was among the first inorganic hole-transporting materials that were introduced early on in metal halide perovskite solar cells, but its full potential as a semiconductor material is still to be realized. We have now performed ultrafast spectroelectrochemical experiments on ITO/CuI electrodes to show the effect of applied bias on the excited-state dynamics in CuI. Under operating conditions, the recombination of excitons is dependent on the applied bias, and it can be accelerated by decreasing the potential from +0.6 to −0.1 V vs Ag/AgCl. Prebiasing experiments show the persistent and reversible “memory” effect of electrochemical bias on charge carrier lifetimes. The excitation of CuI in a CuI/CsPbBr3 film provides synergy between both CuI and CsPbBr3 in dictating the charge separation and recombination.
Owing to its high hole conductivity and ease of preparation, CuI was among the first inorganic hole-transporting materials that were introduced early on in metal halide perovskite solar cells, but its full potential as a semiconductor material is still to be realized. We have now performed ultrafast spectroelectrochemical experiments on ITO/CuI electrodes to show the effect of applied bias on the excited-state dynamics in CuI. Under operating conditions, the recombination of excitons is dependent on the applied bias, and it can be accelerated by decreasing the potential from +0.6 to −0.1 V vs Ag/AgCl. Prebiasing experiments show the persistent and reversible “memory” effect of electrochemical bias on charge carrier lifetimes. The excitation of CuI in a CuI/CsPbBr3 film provides synergy between both CuI and CsPbBr3 in dictating the charge separation and recombination.
524. Influence of Plasmonic CuxS Interfacing Layer on Photovoltaic Performance of CIZS Quantum Dot Sensitized Solar Cells Senthilkumar Muthu, Gary Zaiats, Moorthy Babu Sridharan and Prashant V. Kamat J. Electrochem. Soc. 2019 volume 166, issue 5, H3133-H3137.
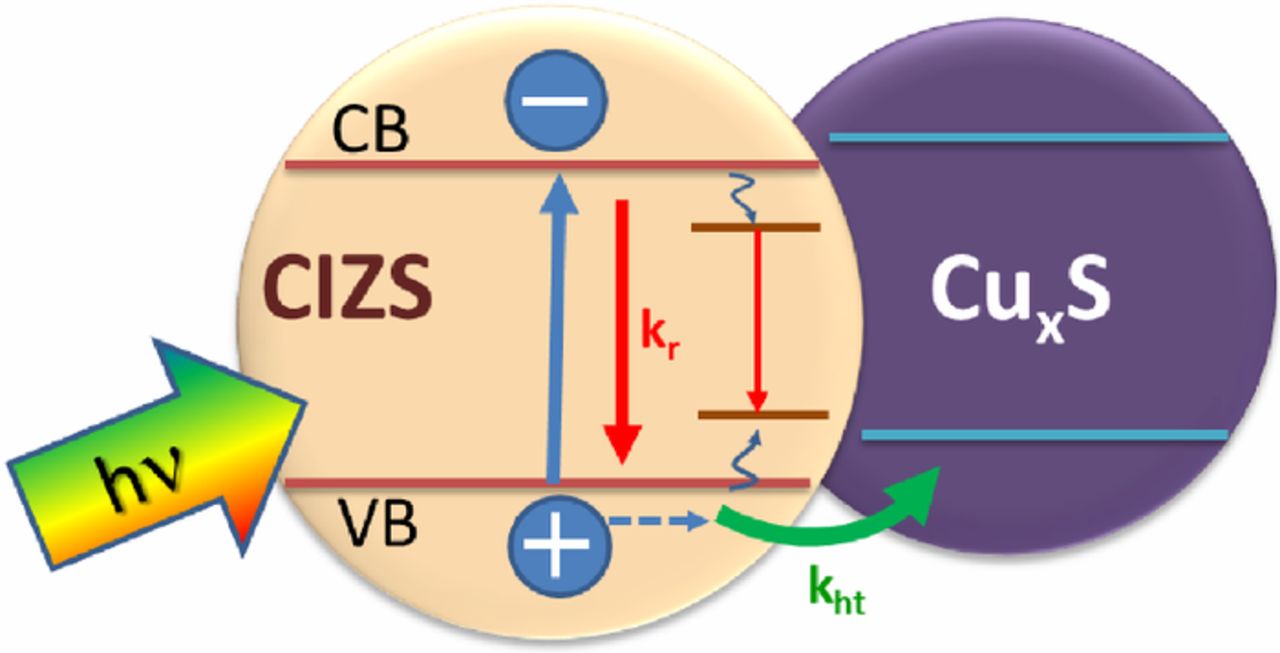 Charge transfer at the semiconductor/electrolyte interface is an important process that dictates the efficiency of quantum dots solar cells. Hole transfer kinetics remains a sluggish reaction for metal chalcogenide electrodes. In this work we examine the beneficial effect of CuxS nanoparticles on the photoelectrochemical performance of Cu-In-Zn-S (CIZS) quantum dots sensitized solar cells as they promote hole transfer to the S2−/Sn2− redox couple. CuxS nanoparticles if deposited without a protecting layer undergo compositional transformation in sulfur rich electrolytes. Addition of a protecting ZnS layer improves the stability and efficiency of the resulting solar cells. Establishing the hole transport property of CuxS in solar cell offers new ways to improve photovoltaic performance of QDSSC.
Charge transfer at the semiconductor/electrolyte interface is an important process that dictates the efficiency of quantum dots solar cells. Hole transfer kinetics remains a sluggish reaction for metal chalcogenide electrodes. In this work we examine the beneficial effect of CuxS nanoparticles on the photoelectrochemical performance of Cu-In-Zn-S (CIZS) quantum dots sensitized solar cells as they promote hole transfer to the S2−/Sn2− redox couple. CuxS nanoparticles if deposited without a protecting layer undergo compositional transformation in sulfur rich electrolytes. Addition of a protecting ZnS layer improves the stability and efficiency of the resulting solar cells. Establishing the hole transport property of CuxS in solar cell offers new ways to improve photovoltaic performance of QDSSC.
523. Optoelectronic Properties of CuI Photoelectrodes Ádám Balog, Gergely F. Samu , Prashant V. Kamat , and Csaba Janáky J. Phys. Chem. Lett. 2019, 10 (2), pp 259–264.
 Detailed mechanistic understanding of the optoelectronic features is a key factor in designing efficient and stable photoelectrodes. In situ spectroelectrochemical methods were employed to scrutinize the effect of trap states on the optical and electronic properties of CuI photoelectrodes and to assess their stability against (photo)electrochemical corrosion. The excitonic band in the absorption spectrum and the Raman spectral features were directly influenced by the applied bias potential. These spectral changes exhibit a good correlation with the alterations observed in the charge-transfer resistance. Interestingly, the population and depopulation of the trap states, which are responsible for the changes in both the optical and electronic properties, occur in a different potential/energy regime. Although cathodic photocorrosion of CuI is thermodynamically favored, this process is kinetically hindered, thus providing good stability in photoelectrochemical operation.
Detailed mechanistic understanding of the optoelectronic features is a key factor in designing efficient and stable photoelectrodes. In situ spectroelectrochemical methods were employed to scrutinize the effect of trap states on the optical and electronic properties of CuI photoelectrodes and to assess their stability against (photo)electrochemical corrosion. The excitonic band in the absorption spectrum and the Raman spectral features were directly influenced by the applied bias potential. These spectral changes exhibit a good correlation with the alterations observed in the charge-transfer resistance. Interestingly, the population and depopulation of the trap states, which are responsible for the changes in both the optical and electronic properties, occur in a different potential/energy regime. Although cathodic photocorrosion of CuI is thermodynamically favored, this process is kinetically hindered, thus providing good stability in photoelectrochemical operation.
2018
522. Glutathione-capped gold nanoclusters: photoinduced energy transfer and singlet oxygen generation Christian Talavera, Prashant V Kamat J. Chem. Sci. (2018) 130: 143.
 Glutathione-capped gold clusters prepared in an aqueous medium are known to exhibit excellent photosensitizing properties. We have now successfully transferred these gold clusters in an organic medium while retaining all the characteristic excited state properties. These gold clusters can be further modified with organic ligands such as 2-Phenylethanethiol (PET). The gold clusters in organic medium exhibit enhanced emission yield ( Φf=0.15 ) compared to that in an aqueous medium ( Φf=0.08 ). The excited state lifetimes of 3.7μs (untreated) and 1.5μs (PET treated) in toluene are also greater than the lifetime observed in aqueous solution ( 0.77μs ). By employing laser flash photolysis we are able to induce triplet energy transfer to β -carotene and oxygen. A singlet oxygen generation with the efficiency of 13% was observed in these experiments. The excited state properties of glutathione-capped gold clusters further shows its importance as a photosensitizer in light energy conversion and biomedical applications.
Glutathione-capped gold clusters prepared in an aqueous medium are known to exhibit excellent photosensitizing properties. We have now successfully transferred these gold clusters in an organic medium while retaining all the characteristic excited state properties. These gold clusters can be further modified with organic ligands such as 2-Phenylethanethiol (PET). The gold clusters in organic medium exhibit enhanced emission yield ( Φf=0.15 ) compared to that in an aqueous medium ( Φf=0.08 ). The excited state lifetimes of 3.7μs (untreated) and 1.5μs (PET treated) in toluene are also greater than the lifetime observed in aqueous solution ( 0.77μs ). By employing laser flash photolysis we are able to induce triplet energy transfer to β -carotene and oxygen. A singlet oxygen generation with the efficiency of 13% was observed in these experiments. The excited state properties of glutathione-capped gold clusters further shows its importance as a photosensitizer in light energy conversion and biomedical applications.
521. Interfacial Charge Transfer between Excited CsPbBr3 Nanocrystals and TiO2: Charge Injection versus Photodegradation Rebecca A. Scheidt, Elisabeth Kerns, and Prashant V. Kamat J. Phys. Chem. Lett. 2018, 9, 20, 5962-5969
 Record-breaking efficiency achieved with quantum dot solar cells made of perovskite nanocrystals demands understanding of the excited-state interactions between perovskite nanocrystals and metal oxide electron transport layers. The interfacial electron transfer between excited CsPbBr3 perovskite nanocrystals and metal oxides (TiO2, SnO2, and ZnO) was elucidated using transient absorption spectroscopy and found to occur with a rate constant in the range of 2–4 × 1010 s–1. In an inert atmosphere, back electron transfer helps to maintain the stability of the perovskite nanocrystals. However, the presence of oxygen introduces instability as it scavenges away transferred electrons from the electron-transporting metal oxide, leaving behind holes to accumulate at CsPbBr3 nanocrystals, which in turn induce anodic corrosion. X-ray photoelectron spectroscopy measurements have enabled us to identify PbO as the major photodegraded product. The importance of the surrounding atmosphere and the supporting metal oxide in governing the stability of perovskite nanocrystals is discussed.
Record-breaking efficiency achieved with quantum dot solar cells made of perovskite nanocrystals demands understanding of the excited-state interactions between perovskite nanocrystals and metal oxide electron transport layers. The interfacial electron transfer between excited CsPbBr3 perovskite nanocrystals and metal oxides (TiO2, SnO2, and ZnO) was elucidated using transient absorption spectroscopy and found to occur with a rate constant in the range of 2–4 × 1010 s–1. In an inert atmosphere, back electron transfer helps to maintain the stability of the perovskite nanocrystals. However, the presence of oxygen introduces instability as it scavenges away transferred electrons from the electron-transporting metal oxide, leaving behind holes to accumulate at CsPbBr3 nanocrystals, which in turn induce anodic corrosion. X-ray photoelectron spectroscopy measurements have enabled us to identify PbO as the major photodegraded product. The importance of the surrounding atmosphere and the supporting metal oxide in governing the stability of perovskite nanocrystals is discussed.
520. Mixed Halide Perovskite Solar Cells. Consequence of Iodide Treatment on Phase Segregation Recovery R. Geetha Balakrishna, Steven M. Kobosko, and Prashant V. Kamat ACS Energy Lett. 2018, 3, 9, 2267-2272
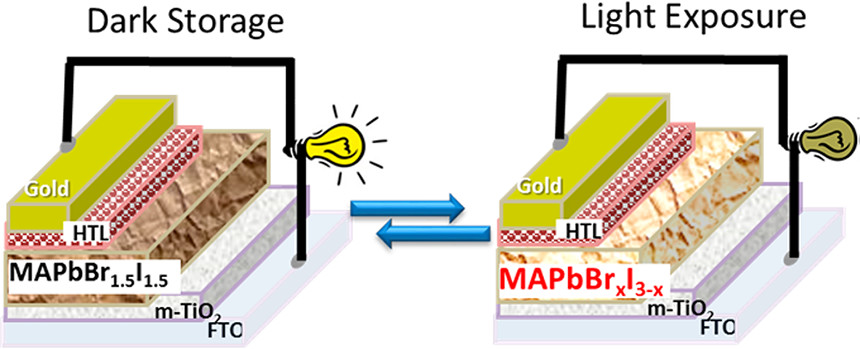 Photoinduced halide ion segregation in mixed halide perovskites can introduce a detrimental effect on the photovoltaic performance of perovskite solar cells over extended light exposure. The photoconversion efficiency decreases from 5.5 to 2.3% when methylammonium lead mixed halide perovskite (MAPbBr1.5I1.5) solar cells are exposed to 25 min of continuous illumination with visible light. The formation of iodide-rich and bromide rich-regions creates traps, which serve as bottlenecks to the flow of charge carriers. Iodide treatment overcomes some of these effects and stabilizes the solar cell performance. It also facilitates quicker recovery in the dark. The decrease in open-circuit voltage following the exposure of visible light was minimal in iodide-treated solar cells. Transient absorption measurements provide insight into the influence of light exposure on charge carrier recombination processes in mixed halide perovskite films. The iodide treatment strategy presented here can aid in designing stable mixed halide perovskite solar cells and the development of multijunction solar cells.
Photoinduced halide ion segregation in mixed halide perovskites can introduce a detrimental effect on the photovoltaic performance of perovskite solar cells over extended light exposure. The photoconversion efficiency decreases from 5.5 to 2.3% when methylammonium lead mixed halide perovskite (MAPbBr1.5I1.5) solar cells are exposed to 25 min of continuous illumination with visible light. The formation of iodide-rich and bromide rich-regions creates traps, which serve as bottlenecks to the flow of charge carriers. Iodide treatment overcomes some of these effects and stabilizes the solar cell performance. It also facilitates quicker recovery in the dark. The decrease in open-circuit voltage following the exposure of visible light was minimal in iodide-treated solar cells. Transient absorption measurements provide insight into the influence of light exposure on charge carrier recombination processes in mixed halide perovskite films. The iodide treatment strategy presented here can aid in designing stable mixed halide perovskite solar cells and the development of multijunction solar cells.
519. Hierarchical Arrays of Cesium Lead Halide Perovskite Nanocrystals through Electrophoretic Deposition Vikash Kumar Ravi, Rebecca A. Scheidt, Jeffrey DuBose, and Prashant V. Kamat J. Am. Chem. Soc. 2018, 140, 28, 8887-8894
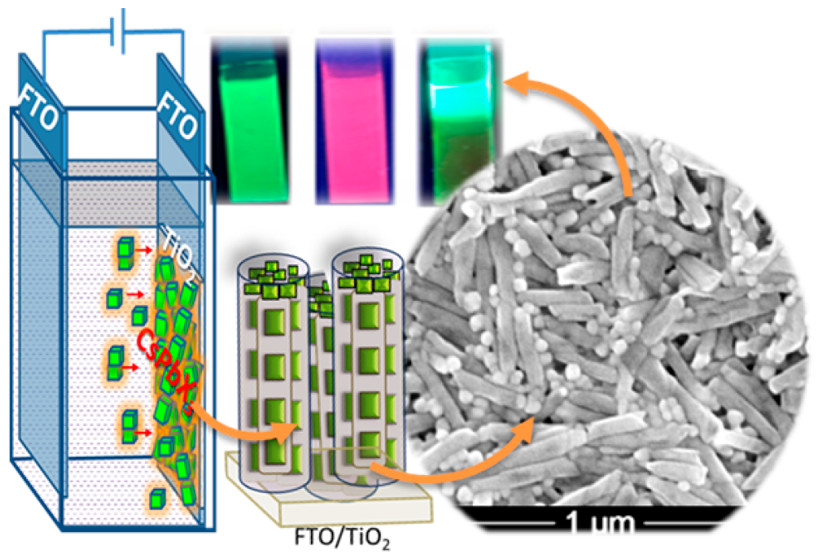 The suppression of halide ion exchange between CsPbBr3 and CsPbI3 nanocrystals achieved through capping with PbSO4–oleate has enabled us to deposit different perovskite nanocrystals as aligned arrays on the electrode surfaces without intermixing of species. The electrophoretic deposition of PbSO4–oleate-capped CsPbX3 (X = Cl, Br, I) nanocrystals suspended in hexane solution on mesoscopic TiO2 films allows the design of controlled architecture with single or multiple layers of perovskite films. The hierarchy in the assembly of these nanocrystals is seen first through the linearly organized nanocrystals in hexane followed by the deposition of larger linear rods ∼500 nm in length. Since most of the photophysical properties of nanocrystals are retained in these aligned arrays, we can design films with tunable luminescence including white color. The electrophoretic deposition of layered films of perovskites in a controlled fashion opens up new ways to design tandem perovskite solar cells and tunable display devices.
The suppression of halide ion exchange between CsPbBr3 and CsPbI3 nanocrystals achieved through capping with PbSO4–oleate has enabled us to deposit different perovskite nanocrystals as aligned arrays on the electrode surfaces without intermixing of species. The electrophoretic deposition of PbSO4–oleate-capped CsPbX3 (X = Cl, Br, I) nanocrystals suspended in hexane solution on mesoscopic TiO2 films allows the design of controlled architecture with single or multiple layers of perovskite films. The hierarchy in the assembly of these nanocrystals is seen first through the linearly organized nanocrystals in hexane followed by the deposition of larger linear rods ∼500 nm in length. Since most of the photophysical properties of nanocrystals are retained in these aligned arrays, we can design films with tunable luminescence including white color. The electrophoretic deposition of layered films of perovskites in a controlled fashion opens up new ways to design tandem perovskite solar cells and tunable display devices.
518. Electrodeposition of Hole-Transport Layer on Methylammonium Lead Iodide Film: A Strategy To Assemble Perovskite Solar Cells Gergely F. Samu, Rebecca A. Scheidt, Gary Zaiats, Prashant V. Kamat, and Csaba Janáky Chem. Mater. 2018, 30, 13, 4202-4206
 The electrochemical deposition of PEDOT offers a convenient way to deposit a hole-transport layer on a MAPbI3 layer for designing n−i−p junction perovskite solar cells. By employing potentiostatically controlled electrodeposition technique, it is possible to obtain controlled thicknesses of HTM layers. An electrochemical post reduction step introduced to control the doping level of the as-deposited PEDOT films is an essential step in achieving better performance of PSCs. Care should be exercised not to destroy the underlying MAPbI3 layer during the post-treatment process. The champion device showed a power conversion efficiency of 5.9%. The results presented in this study open new opportunities to employ electrochemistry to assemble complex architectures of optically active perovskites.
The electrochemical deposition of PEDOT offers a convenient way to deposit a hole-transport layer on a MAPbI3 layer for designing n−i−p junction perovskite solar cells. By employing potentiostatically controlled electrodeposition technique, it is possible to obtain controlled thicknesses of HTM layers. An electrochemical post reduction step introduced to control the doping level of the as-deposited PEDOT films is an essential step in achieving better performance of PSCs. Care should be exercised not to destroy the underlying MAPbI3 layer during the post-treatment process. The champion device showed a power conversion efficiency of 5.9%. The results presented in this study open new opportunities to employ electrochemistry to assemble complex architectures of optically active perovskites.
517. Indium-Rich AgInS2–ZnS Quantum Dots—Ag-/Zn-Dependent Photophysics and Photovoltaics Steven M. Kobosko and Prashant V. Kamat J. Phys. Chem. C 2018, 122, 26, 14336-14344
 AgInS2–ZnS solid solution quantum dots (QDs) prepared with varying Ag/Zn ratios demonstrate composition-dependent photophysical properties. Absorption and emission processes are extremely complex in these compounds because of easily formed crystallographic defects which serve as intraband gap states and provide additional excitation and relaxation pathways. In addition to valence to conduction band absorption, defect states located within the band gap are responsible for tail absorption in these nanoparticles and are assigned to AgIn antisite defects. These AgInS2–ZnS QDs display wavelength-dependent photoluminescence (PL) decays along with large Stokes shifts and long PL lifetimes, strongly suggesting that donor–acceptor pair recombination is the dominant radiative pathway. The excited-state interaction between AgInS2–ZnS and TiO2 is studied through the use of transient absorption spectroscopy, and a fast photoinduced electron-transfer rate constant of 5 × 1011 s–1 is determined. This interaction with TiO2 is further probed by testing various compositions of AgInS2–ZnS in liquid-junction solar cells, with the optimum device power conversion efficiency reaching 1.83%.
AgInS2–ZnS solid solution quantum dots (QDs) prepared with varying Ag/Zn ratios demonstrate composition-dependent photophysical properties. Absorption and emission processes are extremely complex in these compounds because of easily formed crystallographic defects which serve as intraband gap states and provide additional excitation and relaxation pathways. In addition to valence to conduction band absorption, defect states located within the band gap are responsible for tail absorption in these nanoparticles and are assigned to AgIn antisite defects. These AgInS2–ZnS QDs display wavelength-dependent photoluminescence (PL) decays along with large Stokes shifts and long PL lifetimes, strongly suggesting that donor–acceptor pair recombination is the dominant radiative pathway. The excited-state interaction between AgInS2–ZnS and TiO2 is studied through the use of transient absorption spectroscopy, and a fast photoinduced electron-transfer rate constant of 5 × 1011 s–1 is determined. This interaction with TiO2 is further probed by testing various compositions of AgInS2–ZnS in liquid-junction solar cells, with the optimum device power conversion efficiency reaching 1.83%.
516. To Exchange or Not to Exchange. Suppressing Anion Exchange in Cesium Lead Halide Perovskites with PbSO4–Oleate Capping Vikash Kumar Ravi, Rebecca A. Scheidt, Angshuman Nag, Masaru Kuno, and Prashant V. Kamat ACS Energy Lett. 2018, 3, 4, 1049-1055
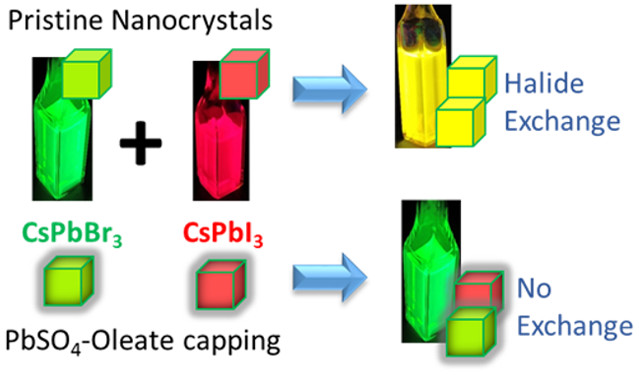 The ease of halide ion exchange in metal halide nanocrystals offers an opportunity to utilize them in a layered or tandem fashion to achieve graded bandgap films. We have now successfully suppressed the halide ion exchange by capping CsPbBr3 and CsPbI3 nanocrystals with PbSO4–oleate to create a nanostructure assembly that inhibits the exchange of anions. Absorption measurements show that the nanocrystal assemblies maintain their identity as either CsPbBr3 or CsPbI3 for several days. Furthermore, the effect of PbSO4–oleate capping on the excited state dynamics has also been elucidated. The effectiveness of PbSO4–oleate capping of lead halide perovskite nanocrystals offers new opportunities to overcome the challenges of halide ion exchange and aid toward the tandem design of perovskite light-harvesting assemblies.
The ease of halide ion exchange in metal halide nanocrystals offers an opportunity to utilize them in a layered or tandem fashion to achieve graded bandgap films. We have now successfully suppressed the halide ion exchange by capping CsPbBr3 and CsPbI3 nanocrystals with PbSO4–oleate to create a nanostructure assembly that inhibits the exchange of anions. Absorption measurements show that the nanocrystal assemblies maintain their identity as either CsPbBr3 or CsPbI3 for several days. Furthermore, the effect of PbSO4–oleate capping on the excited state dynamics has also been elucidated. The effectiveness of PbSO4–oleate capping of lead halide perovskite nanocrystals offers new opportunities to overcome the challenges of halide ion exchange and aid toward the tandem design of perovskite light-harvesting assemblies.
515. Thiolated Gold Nanoclusters for Light Energy Conversion (Review) Muhammad A. Abbas, Prashant V. Kamat, and Jin Ho Bang ACS Energy Lett. 2018, 3, 4, 840-854
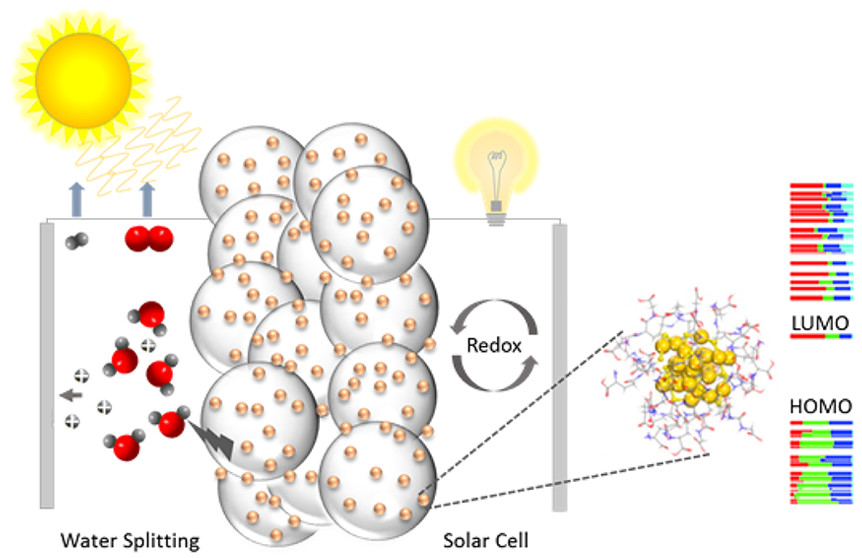 Few-atom gold nanoclusters (NCs) exhibit molecule-like properties due to a discrete electronic structure driven by the quantum confinement effect. Unlike plasmonic Au particles, these nonplasmonic particles of diameter less than 2 nm, commonly referred to as nanoclusters, possess a distinct excited-state behavior that can offer a new opportunity to employ them as a photosensitizer. Their size-dependent excited-state behavior enables establishing logical designing principles to build up efficient light energy conversion systems. The photodynamics of thiolated Au NCs and efforts to exploit the Au NCs in light energy conversion applications discussed in this Review show new opportunities to utilize them as photosensitizers. Current bottlenecks in implementing thiolated Au NCs in light conversion applications and new strategies and future directions to address these limitations are also discussed.
Few-atom gold nanoclusters (NCs) exhibit molecule-like properties due to a discrete electronic structure driven by the quantum confinement effect. Unlike plasmonic Au particles, these nonplasmonic particles of diameter less than 2 nm, commonly referred to as nanoclusters, possess a distinct excited-state behavior that can offer a new opportunity to employ them as a photosensitizer. Their size-dependent excited-state behavior enables establishing logical designing principles to build up efficient light energy conversion systems. The photodynamics of thiolated Au NCs and efforts to exploit the Au NCs in light energy conversion applications discussed in this Review show new opportunities to utilize them as photosensitizers. Current bottlenecks in implementing thiolated Au NCs in light conversion applications and new strategies and future directions to address these limitations are also discussed.
514. Probing Interfacial Electrochemistry on a Co3O4 Water Oxidation Catalyst Using Lab-Based Ambient Pressure X-ray Photoelectron Spectroscopy Xueqiang Zhang, Yong-Siou Chen, Prashant V. Kamat, and Sylwia Ptasinska J. Phys. Chem. C 2018, 122, 25, 13894-13901
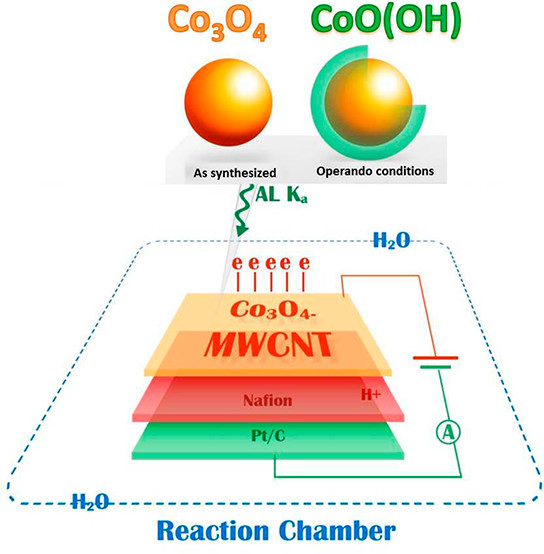 The design and mechanistic understanding of efficient and low-cost catalysts for the oxygen evolution reaction (OER) are currently the focus of electrochemical water-splitting technology. Herein, we report the chemical transformations on the water-vapor/solid interface and catalytic performance of an OER catalyst consisting of Co3O4 nanoparticles on multiwalled carbon nanotubes (Co3O4–MWCNT). Using a specially constructed electrochemical cell incorporated to the lab-based ambient-pressure X-ray photoelectron spectroscopy (APXPS) to mimic operando conditions, we obtained experimental evidence for the formation of CoO(OH) as the catalytically active phase on a Co3O4–MWCNT OER catalyst. Under water and applied potential conditions, CoO(OH) is formed, enriching the surface of Co3O4 nanoparticles with subnanometer thickness, and oxidizing H2O into O2. However, immediately after the removal of the applied potential, the CoO(OH) phase is converted back to Co3O4. This back-conversion from CoO(OH) to Co3O4 is likely driven by locally concentrated protons (H+) in water vapor, which shows the necessity of an electrochemical bias to preserve the catalytically active phase. These results reveal the surface chemical identities of the Co3O4–MWCNT OER catalyst, which are in agreement with those obtained from in-situ APXPS studies of liquid/solid interfaces consisting of Co3O4 catalyst and disagree with those obtained from ex-situ ultrahigh vacuum (UHV) XPS. Thus, our results demonstrate the possibility of performing surface chemical analysis in simplified electrochemical systems and further reinforce the importance of performing mechanistic studies of electrochemical devices under in-situ conditions.
The design and mechanistic understanding of efficient and low-cost catalysts for the oxygen evolution reaction (OER) are currently the focus of electrochemical water-splitting technology. Herein, we report the chemical transformations on the water-vapor/solid interface and catalytic performance of an OER catalyst consisting of Co3O4 nanoparticles on multiwalled carbon nanotubes (Co3O4–MWCNT). Using a specially constructed electrochemical cell incorporated to the lab-based ambient-pressure X-ray photoelectron spectroscopy (APXPS) to mimic operando conditions, we obtained experimental evidence for the formation of CoO(OH) as the catalytically active phase on a Co3O4–MWCNT OER catalyst. Under water and applied potential conditions, CoO(OH) is formed, enriching the surface of Co3O4 nanoparticles with subnanometer thickness, and oxidizing H2O into O2. However, immediately after the removal of the applied potential, the CoO(OH) phase is converted back to Co3O4. This back-conversion from CoO(OH) to Co3O4 is likely driven by locally concentrated protons (H+) in water vapor, which shows the necessity of an electrochemical bias to preserve the catalytically active phase. These results reveal the surface chemical identities of the Co3O4–MWCNT OER catalyst, which are in agreement with those obtained from in-situ APXPS studies of liquid/solid interfaces consisting of Co3O4 catalyst and disagree with those obtained from ex-situ ultrahigh vacuum (UHV) XPS. Thus, our results demonstrate the possibility of performing surface chemical analysis in simplified electrochemical systems and further reinforce the importance of performing mechanistic studies of electrochemical devices under in-situ conditions.
513. A quantitative and spatially resolved analysis of the performance-bottleneck in high efficiency, planar hybrid perovskite solar cells Sergiu Draguta, Jeffrey A. Christians, Yurii V. Morozov, Anselme Mucunzi, Joseph S. Manser, Prashant V. Kamat, Joseph M. Luther and Masaru Kuno Energy Environ. Sci. 2018, 11, 960-969
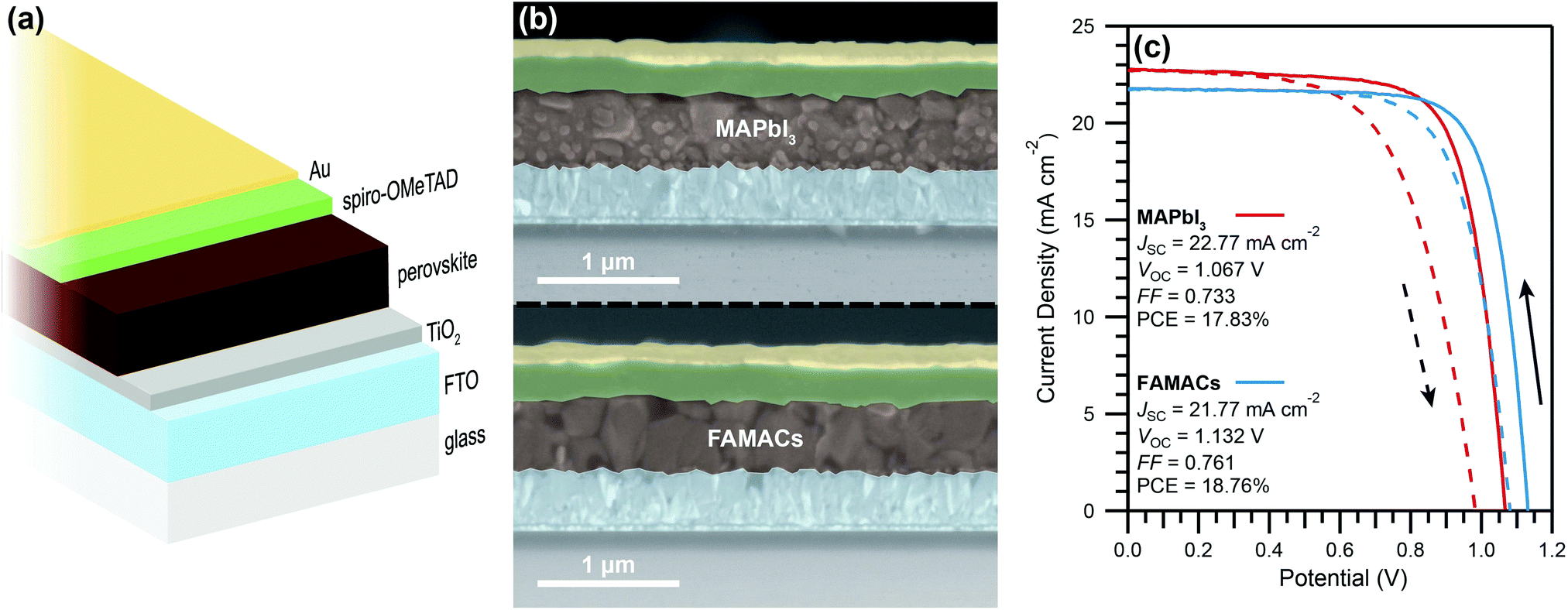 Hybrid perovskites represent a potential paradigm shift for the creation of low-cost solar cells. Current power conversion efficiencies (PCEs) exceed 22%. However, despite this, record PCEs are still far from their theoretical Shockley–Queisser limit of 31%. To increase these PCE values, there is a pressing need to understand, quantify and microscopically model charge recombination processes in full working devices. Here, we present a complete microscopic account of charge recombination processes in high efficiency (18–19% PCE) hybrid perovskite (mixed cation and methylammonium lead iodide) solar cells. We employ diffraction-limited optical measurements along with relevant kinetic modeling to establish, for the first time, local photoluminescence quantum yields, trap densities, trapping efficiencies, charge extraction efficiencies, quasi-Fermi-level splitting, and effective PCE estimates. Correlations between these spatially resolved parameters, in turn, allow us to conclude that intrinsic electron traps in the perovskite active layers limit the performance of these state-of-the-art hybrid perovskite solar cells.
Hybrid perovskites represent a potential paradigm shift for the creation of low-cost solar cells. Current power conversion efficiencies (PCEs) exceed 22%. However, despite this, record PCEs are still far from their theoretical Shockley–Queisser limit of 31%. To increase these PCE values, there is a pressing need to understand, quantify and microscopically model charge recombination processes in full working devices. Here, we present a complete microscopic account of charge recombination processes in high efficiency (18–19% PCE) hybrid perovskite (mixed cation and methylammonium lead iodide) solar cells. We employ diffraction-limited optical measurements along with relevant kinetic modeling to establish, for the first time, local photoluminescence quantum yields, trap densities, trapping efficiencies, charge extraction efficiencies, quasi-Fermi-level splitting, and effective PCE estimates. Correlations between these spatially resolved parameters, in turn, allow us to conclude that intrinsic electron traps in the perovskite active layers limit the performance of these state-of-the-art hybrid perovskite solar cells.
512. Electrochemistry and Spectroelectrochemistry of Lead Halide Perovskite Films: Materials Science Aspects and Boundary Conditions Gergely F. Samu, Rebecca A. Scheidt, Prashant V. Kamat, and Csaba Janáky Chem. Mater. 2018, 30, 3, 561-569
 The unique optoelectronic properties of lead halide perovskites have triggered a new wave of excitement in materials chemistry during the past five years. Electrochemistry, spectroelectrochemistry, and photoelectrochemistry could be viable tools both for analyzing the optoelectronic features of these materials and for assembling them into hybrid architectures (e.g., solar cells). At the same time, the instability of these materials limits the pool of solvents and electrolytes that can be employed in such experiments. The focus of our study is to establish a stability window for electrochemical tests for all-inorganic CsPbBr3 and hybrid organic–inorganic MAPbI3 perovskites. In addition, we aimed to understand the reduction and oxidation events that occur and to assess the damage done during these processes at extreme electrochemical conditions. In this vein, we demonstrated the chemical, structural, and morphological changes of the films in both reductive and oxidative environments. Taking all these results together as a whole, we propose a set of boundary conditions and protocols for how electrochemical experiments with lead halide perovskites should be carried out and interpreted. The presented results will contribute to the understanding of the electrochemical response of these materials and lead to a standardization of results in the literature so that comparisons can more easily be made.
The unique optoelectronic properties of lead halide perovskites have triggered a new wave of excitement in materials chemistry during the past five years. Electrochemistry, spectroelectrochemistry, and photoelectrochemistry could be viable tools both for analyzing the optoelectronic features of these materials and for assembling them into hybrid architectures (e.g., solar cells). At the same time, the instability of these materials limits the pool of solvents and electrolytes that can be employed in such experiments. The focus of our study is to establish a stability window for electrochemical tests for all-inorganic CsPbBr3 and hybrid organic–inorganic MAPbI3 perovskites. In addition, we aimed to understand the reduction and oxidation events that occur and to assess the damage done during these processes at extreme electrochemical conditions. In this vein, we demonstrated the chemical, structural, and morphological changes of the films in both reductive and oxidative environments. Taking all these results together as a whole, we propose a set of boundary conditions and protocols for how electrochemical experiments with lead halide perovskites should be carried out and interpreted. The presented results will contribute to the understanding of the electrochemical response of these materials and lead to a standardization of results in the literature so that comparisons can more easily be made.
511. Modulation of Charge Recombination in CsPbBr3 Perovskite Films with Electrochemical Bias Rebecca A. Scheidt, Gergely F. Samu, Csaba Janáky, and Prashant V. Kamat J. Am. Chem. Soc. 2018, 140, 1, 86-89
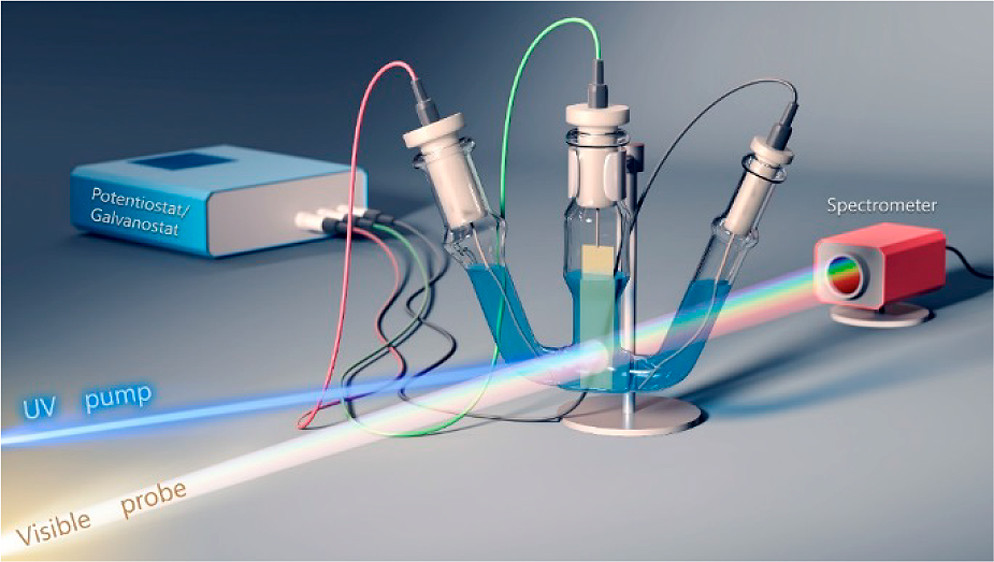 The charging of a mesoscopic TiO2 layer in a metal halide perovskite solar cell can influence the overall power conversion efficiency. By employing CsPbBr3 films deposited on a mesoscopic TiO2 film, we have succeeded in probing the influence of electrochemical bias on the charge carrier recombination process. The transient absorption spectroscopy experiments conducted at different applied potentials indicate a decrease in the charge carrier lifetimes of CsPbBr3 as we increase the potential from −0.6 to +0.6 V vs Ag/AgCl. The charge carrier lifetime increased upon reversing the applied bias, thus indicating the reversibility of the photoresponse to charging effects. The ultrafast spectroelectrochemical experiments described here offer a convenient approach to probe the charging effects in perovskite solar cells.
The charging of a mesoscopic TiO2 layer in a metal halide perovskite solar cell can influence the overall power conversion efficiency. By employing CsPbBr3 films deposited on a mesoscopic TiO2 film, we have succeeded in probing the influence of electrochemical bias on the charge carrier recombination process. The transient absorption spectroscopy experiments conducted at different applied potentials indicate a decrease in the charge carrier lifetimes of CsPbBr3 as we increase the potential from −0.6 to +0.6 V vs Ag/AgCl. The charge carrier lifetime increased upon reversing the applied bias, thus indicating the reversibility of the photoresponse to charging effects. The ultrafast spectroelectrochemical experiments described here offer a convenient approach to probe the charging effects in perovskite solar cells.
510. Ligand Assisted Transformation of Cubic CsPbBr3 Nanocrystals into Two-Dimensional CsPb2Br5 Nanosheets Subila K. Balakrishnan and Prashant V. Kamat Chem. Mater. 2018, 30, 1, 74-78
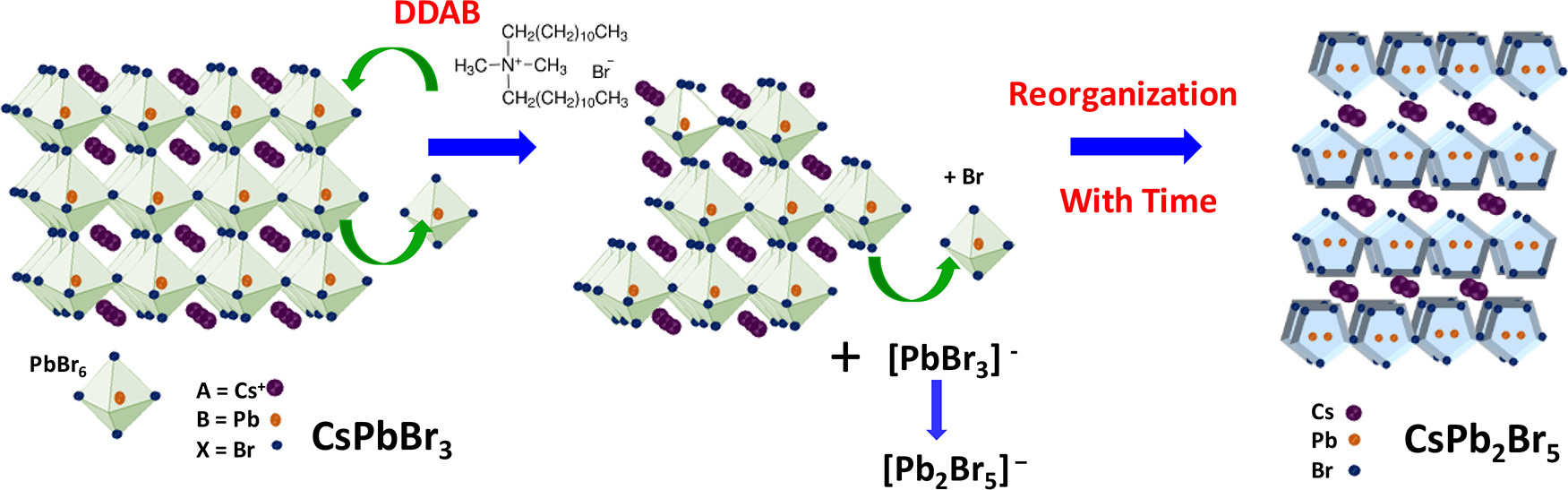 Ligand-assisted transformation of CsPbBr3 NCs to 2-D CsPb2Br5 nanosheets offers a simple technique to alter the morphology of perovskite structures. Such structural transformations are likely to be important for surface treatment to passivate defect sites and design 3-D/2-D interfaces. By subjecting CsPbBr3 films to controlled surface treatment with DDAB it should be possible to create such interfaces. As demonstrated recently, creation of the 3-D/2-D interface improves the photovoltaic and optoelectronic properties of perovskite solar cells.
Ligand-assisted transformation of CsPbBr3 NCs to 2-D CsPb2Br5 nanosheets offers a simple technique to alter the morphology of perovskite structures. Such structural transformations are likely to be important for surface treatment to passivate defect sites and design 3-D/2-D interfaces. By subjecting CsPbBr3 films to controlled surface treatment with DDAB it should be possible to create such interfaces. As demonstrated recently, creation of the 3-D/2-D interface improves the photovoltaic and optoelectronic properties of perovskite solar cells.
2017
509. CsPbBr3 Solar Cells: Controlled Film Growth through Layer-by-Layer Quantum Dot Deposition Jacob B. Hoffman, Gary Zaiats, Isaac Wappes, and Prashant V. Kamat Chem. Mater. 2017, 29, 22, 9767-9774
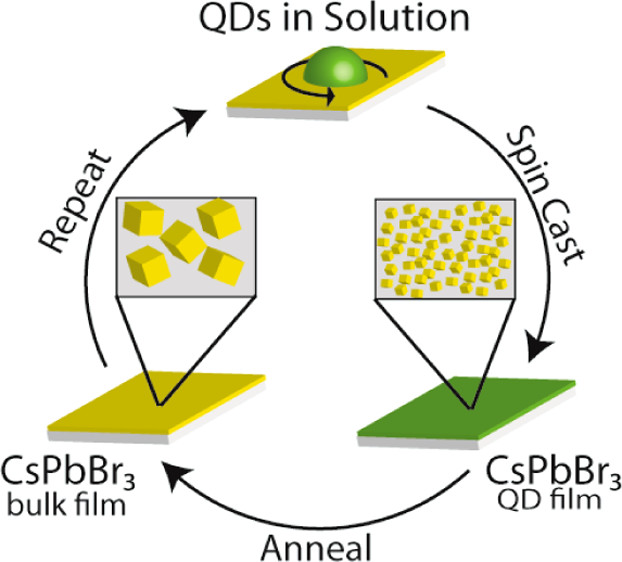 All inorganic cesium lead bromide (CsPbBr3) perovskite is a more stable alternative to methylammonium lead bromide (MAPbBr3) for designing high open-circuit voltage solar cells and display devices. Poor solubility of CsBr in organic solvents makes typical solution deposition methods difficult to adapt for constructing CsPbBr3 devices. Our layer-by-layer methodology, which makes use of CsPbBr3 quantum dot (QD) deposition followed by annealing, provides a convenient way to cast stable films of desired thickness. The transformation from QDs into bulk during thermal annealing arises from the resumption of nanoparticle growth and not from sintering as generally assumed. Additionally, a large loss of organic material during the annealing process is mainly from 1-octadecene left during the QD synthesis. Utilizing this deposition approach for perovskite photovoltaics is examined using typical planar architecture devices. Devices optimized to both QD spin-casting concentration and overall CsPbBr3 thickness produce champion devices that reach power conversion efficiencies of 5.5% with a Voc value of 1.4 V. The layered QD deposition demonstrates a controlled perovskite film architecture for developing efficient, high open-circuit photovoltaic devices.
All inorganic cesium lead bromide (CsPbBr3) perovskite is a more stable alternative to methylammonium lead bromide (MAPbBr3) for designing high open-circuit voltage solar cells and display devices. Poor solubility of CsBr in organic solvents makes typical solution deposition methods difficult to adapt for constructing CsPbBr3 devices. Our layer-by-layer methodology, which makes use of CsPbBr3 quantum dot (QD) deposition followed by annealing, provides a convenient way to cast stable films of desired thickness. The transformation from QDs into bulk during thermal annealing arises from the resumption of nanoparticle growth and not from sintering as generally assumed. Additionally, a large loss of organic material during the annealing process is mainly from 1-octadecene left during the QD synthesis. Utilizing this deposition approach for perovskite photovoltaics is examined using typical planar architecture devices. Devices optimized to both QD spin-casting concentration and overall CsPbBr3 thickness produce champion devices that reach power conversion efficiencies of 5.5% with a Voc value of 1.4 V. The layered QD deposition demonstrates a controlled perovskite film architecture for developing efficient, high open-circuit photovoltaic devices.
508. Quantum Dot Light-Emitting Devices: Beyond Alignment of Energy Levels Gary Zaiats, Shingo Ikeda, Sachin Kinge, and Prashant V. Kamat ACS Appl. Mater. Interfaces 2017, 9, 36, 30741-30745
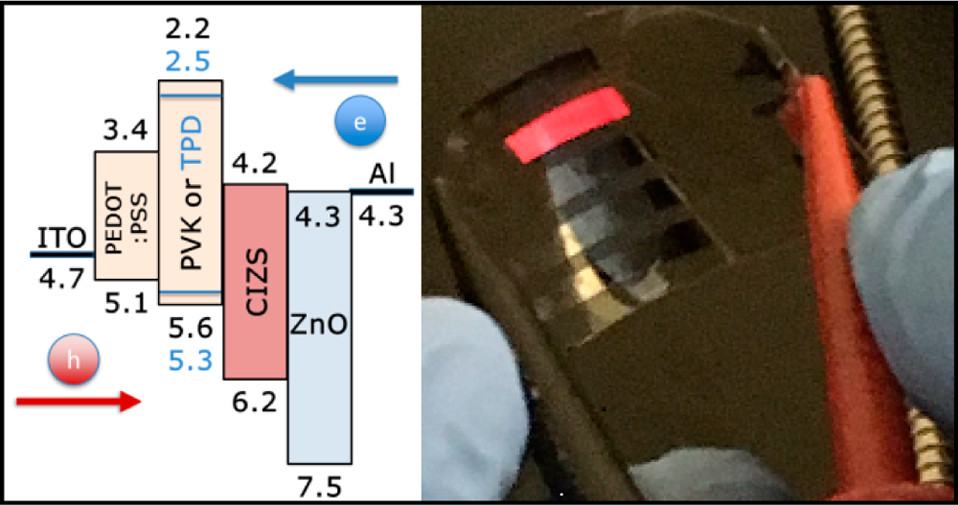 Multinary semiconductor nanoparticles such as CuInS2, AgInS2, and the corresponding alloys with ZnS hold promise for designing future quantum dot light-emitting devices (QLED). The QLED architectures require matching of energy levels between the different electron and hole transport layers. In addition to energy level alignment, conductivity and charge transfer interactions within these layers determine the overall efficiency of QLED. By employing CuInS2–ZnS QDs we succeeded in fabricating red-emitting QLED using two different hole-transporting materials, polyvinylcarbazole and poly(4-butylphenyldiphenylamine). Despite the similarity of the HOMO–LUMO energy levels of these two hole transport materials, the QLED devices exhibit distinctly different voltage dependence. The difference in onset voltage and excited state interactions shows the complexity involved in selecting the hole transport materials for display devices.
Multinary semiconductor nanoparticles such as CuInS2, AgInS2, and the corresponding alloys with ZnS hold promise for designing future quantum dot light-emitting devices (QLED). The QLED architectures require matching of energy levels between the different electron and hole transport layers. In addition to energy level alignment, conductivity and charge transfer interactions within these layers determine the overall efficiency of QLED. By employing CuInS2–ZnS QDs we succeeded in fabricating red-emitting QLED using two different hole-transporting materials, polyvinylcarbazole and poly(4-butylphenyldiphenylamine). Despite the similarity of the HOMO–LUMO energy levels of these two hole transport materials, the QLED devices exhibit distinctly different voltage dependence. The difference in onset voltage and excited state interactions shows the complexity involved in selecting the hole transport materials for display devices.
507. Revival of Solar Paint Concept: Air-Processable Solar Paints for the Fabrication of Quantum Dot-Sensitized Solar Cells Muhammad A. Abbas, Muhammad A. Basit, Seog Joon Yoon, Geun Jun Lee, Moo Dong Lee, Tae Joo Park, Prashant V. Kamat, and Jin Ho Bang J. Phys. Chem. C 2017, 121, 33, 17658-17670
 One way to revolutionize solar energy production and expand it to a large scale is to reduce the manufacturing cost and complexity of the fabrication process. The ability to make solar cells on the surface of any shape would further transform this technology. Quantum dot-sensitized solar cells (QDSSCs) are an ideal candidate to push solar cell technology in this direction. In this regard, making a paint that can be applied by a paint brush to any transparent conductive surface to turn it into the photoanode of QDSSCs is the ultimate goal. We herein demonstrate the feasibility of one-coat fabrication of QDSSCs from a lead sulfide (PbS)-based solar paint. This is possible because of its unique ability to regenerate after oxidation occurred during heat treatment in air. Hence, the whole fabrication process can be carried out in air unlike a first-generation solar paint based on cadmium sulfide (CdS) and cadmium selenide (CdSe). Two solar paints using a commercially available titanium dioxide (TiO2) and a p-type TiO2 powder were synthesized and evaluated. Also, the performance-limiting parameters are thoroughly investigated using various spectroscopic and electrochemical characterization methods. The implication of new insights into the PbS-based solar paint for further development of paint-on solar cells is discussed.
One way to revolutionize solar energy production and expand it to a large scale is to reduce the manufacturing cost and complexity of the fabrication process. The ability to make solar cells on the surface of any shape would further transform this technology. Quantum dot-sensitized solar cells (QDSSCs) are an ideal candidate to push solar cell technology in this direction. In this regard, making a paint that can be applied by a paint brush to any transparent conductive surface to turn it into the photoanode of QDSSCs is the ultimate goal. We herein demonstrate the feasibility of one-coat fabrication of QDSSCs from a lead sulfide (PbS)-based solar paint. This is possible because of its unique ability to regenerate after oxidation occurred during heat treatment in air. Hence, the whole fabrication process can be carried out in air unlike a first-generation solar paint based on cadmium sulfide (CdS) and cadmium selenide (CdSe). Two solar paints using a commercially available titanium dioxide (TiO2) and a p-type TiO2 powder were synthesized and evaluated. Also, the performance-limiting parameters are thoroughly investigated using various spectroscopic and electrochemical characterization methods. The implication of new insights into the PbS-based solar paint for further development of paint-on solar cells is discussed.
506. Rationalizing the light-induced phase separation of mixed halide organic–inorganic perovskites Sergiu Draguta, Onise Sharia, Seog Joon Yoon, Michael C. Brennan, Yurii V. Morozov, Joseph S. Manser, Prashant V. Kamat, William F. Schneider & Masaru Kuno Nat. Commun. 2017, 2, 8, 1860-1861
 Mixed halide hybrid perovskites, CH3NH3Pb(I1−xBrx)3, represent good candidates for low-cost, high efficiency photovoltaic, and light-emitting devices. Their band gaps can be tuned from 1.6 to 2.3 eV, by changing the halide anion identity. Unfortunately, mixed halide perovskites undergo phase separation under illumination. This leads to iodide- and bromide-rich domains along with corresponding changes to the material’s optical/electrical response. Here, using combined spectroscopic measurements and theoretical modeling, we quantitatively rationalize all microscopic processes that occur during phase separation. Our model suggests that the driving force behind phase separation is the bandgap reduction of iodide-rich phases. It additionally explains observed non-linear intensity dependencies, as well as self-limited growth of iodide-rich domains. Most importantly, our model reveals that mixed halide perovskites can be stabilized against phase separation by deliberately engineering carrier diffusion lengths and injected carrier densities.
Mixed halide hybrid perovskites, CH3NH3Pb(I1−xBrx)3, represent good candidates for low-cost, high efficiency photovoltaic, and light-emitting devices. Their band gaps can be tuned from 1.6 to 2.3 eV, by changing the halide anion identity. Unfortunately, mixed halide perovskites undergo phase separation under illumination. This leads to iodide- and bromide-rich domains along with corresponding changes to the material’s optical/electrical response. Here, using combined spectroscopic measurements and theoretical modeling, we quantitatively rationalize all microscopic processes that occur during phase separation. Our model suggests that the driving force behind phase separation is the bandgap reduction of iodide-rich phases. It additionally explains observed non-linear intensity dependencies, as well as self-limited growth of iodide-rich domains. Most importantly, our model reveals that mixed halide perovskites can be stabilized against phase separation by deliberately engineering carrier diffusion lengths and injected carrier densities.
505. A Victim of Halide Ion Segregation. How Light Soaking Affects Solar Cell Performance of Mixed Halide Lead Perovskites Gergely F. Samu, Csaba Janáky, and Prashant V. Kamat ACS Energy Lett. 2017, 2, 8, 1860-1861
 Photoinduced segregation in mixed halide perovskites has a direct influence on decreasing the solar cell efficiency as segregated I-rich domains serve as charge recombination centers. The changes in the external quantum efficiency mirror the spectral loss in the absorption; however, the time scale of the IPCE recovery in the dark is slower than the absorption recovery, showing the intricate nature of the photoinduced halide segregation and charge collection in solar cell devices.
Photoinduced segregation in mixed halide perovskites has a direct influence on decreasing the solar cell efficiency as segregated I-rich domains serve as charge recombination centers. The changes in the external quantum efficiency mirror the spectral loss in the absorption; however, the time scale of the IPCE recovery in the dark is slower than the absorption recovery, showing the intricate nature of the photoinduced halide segregation and charge collection in solar cell devices.
504. Shift Happens. How Halide Ion Defects Influence Photoinduced Segregation in Mixed Halide Perovskites Seog Joon Yoon, Masaru Kuno, and Prashant V. Kamat ACS Energy Lett. 2017, 2, 7, 1507-1514
 Minimizing photoinduced segregation in mixed halide lead perovskites is important for achieving stable photovoltaic performance. The shift in the absorption and the rate of formation of iodide- and bromide-rich regions following visible excitation of mixed halide lead perovskites is found to strongly depend on the halide ion concentration. Slower formation and recovery rates observed in halide-deficient films indicate the involvement of defect sites in influencing halide phase segregation. At higher halide concentrations (in stoichiometric excess), segregation effects become less prominent, as evidenced by faster recovery kinetics. These results suggest that light-induced compositional segregation can be minimized in mixed halide perovskite films by using excess halide ions. The findings from this study further reflect the importance of halide ion post-treatment of perovskite films to improve their solar cell performance.
Minimizing photoinduced segregation in mixed halide lead perovskites is important for achieving stable photovoltaic performance. The shift in the absorption and the rate of formation of iodide- and bromide-rich regions following visible excitation of mixed halide lead perovskites is found to strongly depend on the halide ion concentration. Slower formation and recovery rates observed in halide-deficient films indicate the involvement of defect sites in influencing halide phase segregation. At higher halide concentrations (in stoichiometric excess), segregation effects become less prominent, as evidenced by faster recovery kinetics. These results suggest that light-induced compositional segregation can be minimized in mixed halide perovskite films by using excess halide ions. The findings from this study further reflect the importance of halide ion post-treatment of perovskite films to improve their solar cell performance.
503. Wavelength-Dependent Ultrafast Charge Carrier Separation in the WO3/BiVO4 Coupled System Ivan Grigioni, Kevin G. Stamplecoskie, Danilo H. Jara, Maria Vittoria Dozzi, Aurelio Oriana, Giulio Cerullo, Prashant V. Kamat, and Elena Selli ACS Energy Lett. 2017, 2, 6, 1362-1367
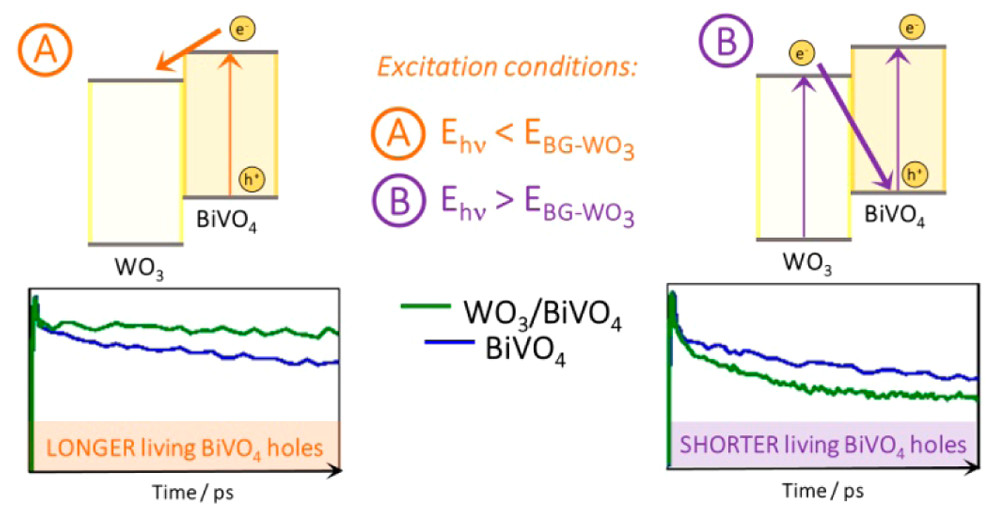 Due to its ~2.4 eV band gap, BiVO4 is a very promising photoanode material for harvesting the blue portion of the solar light for photoelectrochemical (PEC) water splitting applications. In WO3/BiVO4 heterojunction films, the electrons photoexcited in BiVO4 are injected into WO3, overcoming the lower charge carriers’ diffusion properties limiting the PEC performance of BiVO4 photoanodes. Here, we investigate by ultrafast transient absorption spectroscopy the charge carrier interactions occurring at the interface between the two oxides in heterojunction systems to directly unveil their wavelength dependence. Under selective BiVO4 excitation, a favorable electron transfer from photoexcited BiVO4 to WO3 occurs immediately after excitation and leads to an increase of the trapped holes’ lifetime in BiVO4. However, a recombination channel opens when both oxides are simultaneously excited, evidenced by a shorter lifetime of trapped holes in BiVO4. PEC measurements reveal the implication of these wavelength-dependent ultrafast interactions on the performances of the WO3/BiVO4 heterojunction.
Due to its ~2.4 eV band gap, BiVO4 is a very promising photoanode material for harvesting the blue portion of the solar light for photoelectrochemical (PEC) water splitting applications. In WO3/BiVO4 heterojunction films, the electrons photoexcited in BiVO4 are injected into WO3, overcoming the lower charge carriers’ diffusion properties limiting the PEC performance of BiVO4 photoanodes. Here, we investigate by ultrafast transient absorption spectroscopy the charge carrier interactions occurring at the interface between the two oxides in heterojunction systems to directly unveil their wavelength dependence. Under selective BiVO4 excitation, a favorable electron transfer from photoexcited BiVO4 to WO3 occurs immediately after excitation and leads to an increase of the trapped holes’ lifetime in BiVO4. However, a recombination channel opens when both oxides are simultaneously excited, evidenced by a shorter lifetime of trapped holes in BiVO4. PEC measurements reveal the implication of these wavelength-dependent ultrafast interactions on the performances of the WO3/BiVO4 heterojunction.
502. Semiconductor Surface Chemistry as Holy Grail in Photocatalysis and Photovoltaics (Review) Kamat, P. V. Acc. Chem. Res. 2017, 50, 3, 527-531
 The trail of semiconductor surface photochemistry during the past four decades has led to the emergence of new areas in chemistry (e.g., photocatalysis, solar cells, solar fuels). How can one now exploit the richness of surface chemistry of hybrid architectures and make a transformative leap in light energy conversion and other applications?
The trail of semiconductor surface photochemistry during the past four decades has led to the emergence of new areas in chemistry (e.g., photocatalysis, solar cells, solar fuels). How can one now exploit the richness of surface chemistry of hybrid architectures and make a transformative leap in light energy conversion and other applications?
501. AgInS2–ZnS Quantum Dots: Excited State Interactions with TiO2 and Photovoltaic Performance Steven M. Kobosko, Danilo H. Jara, and Prashant V. Kamat ACS Appl. Mater. Interfaces. 2017, 9, 39, 33379-33388
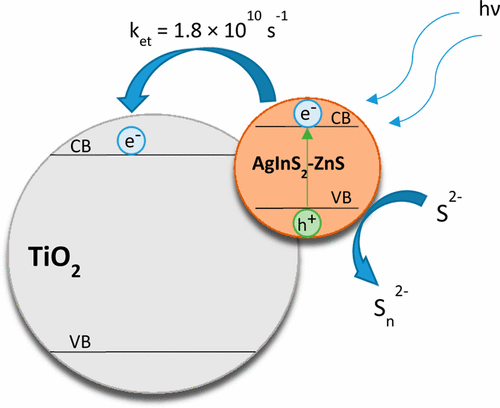 Multinary quantum dots such as AgInS2 and alloyed AgInS2–ZnS are an emerging class of semiconductor materials for applications in photovoltaic and display devices. The nanocrystals of (AgInS2)x–(ZnS)1–x (for x = 0.67) exhibit a broad emission with a maximum at 623 nm and interact strongly with TiO2 nanostructures by injecting electrons from the excited state. The electron transfer rate constant as determined from transient absorption spectroscopy was 1.8 × 1010 s–1. The photovoltaic performance was evaluated over a period of a few weeks to demonstrate the stability of AgInS2–ZnS when utilized as sensitizers in solar cells. We report a power conversion efficiency of 2.25% of our champion cell 1 month after its fabrication. The limitations of AgInS2–ZnS nanocrystals in achieving greater solar cell efficiency are discussed.
Multinary quantum dots such as AgInS2 and alloyed AgInS2–ZnS are an emerging class of semiconductor materials for applications in photovoltaic and display devices. The nanocrystals of (AgInS2)x–(ZnS)1–x (for x = 0.67) exhibit a broad emission with a maximum at 623 nm and interact strongly with TiO2 nanostructures by injecting electrons from the excited state. The electron transfer rate constant as determined from transient absorption spectroscopy was 1.8 × 1010 s–1. The photovoltaic performance was evaluated over a period of a few weeks to demonstrate the stability of AgInS2–ZnS when utilized as sensitizers in solar cells. We report a power conversion efficiency of 2.25% of our champion cell 1 month after its fabrication. The limitations of AgInS2–ZnS nanocrystals in achieving greater solar cell efficiency are discussed.
500. Why Surface Chemistry Matters for QD–QD Resonance Energy Transfer Jacob B. Hoffman, Rabeka Alam, and Prashant V. Kamat ACS Energy Lett. 2017, 2, pp 391–396
 Resonance energy transfer (RET) has been shown to occur in films of semiconductor quantum dots (QDs) with variation in QD composition and size. When coupled with charge carrier transfer, RET could provide a complementary strategy for light harvesting in QD based solid state photovoltaic devices. Due to a direct dependence on the optical properties of the donor and acceptor, QD surface chemistry plays a drastic role in determining the efficiency of RET. Here, the impact of QD surface chemistry on RET in QD films was investigated using a pair of different sized CdSe QDs spin-cast onto a glass substrate. The effects of QD surface passivation on RET were studied by removing surface ligands through QD washing and adding an insulating ZnS shell. In addition, QD films were subjected to solid state ligand exchanges with thiolated ligands in order to mimic a layer-by-layer deposition method commonly used in the construction of QD photovoltaics. These solid state ligand exchanges exhibit drastic quenching of RET in the films. These experiments highlight the importance of understanding surface chemistry when designing photovoltaics that utilize RET.
Resonance energy transfer (RET) has been shown to occur in films of semiconductor quantum dots (QDs) with variation in QD composition and size. When coupled with charge carrier transfer, RET could provide a complementary strategy for light harvesting in QD based solid state photovoltaic devices. Due to a direct dependence on the optical properties of the donor and acceptor, QD surface chemistry plays a drastic role in determining the efficiency of RET. Here, the impact of QD surface chemistry on RET in QD films was investigated using a pair of different sized CdSe QDs spin-cast onto a glass substrate. The effects of QD surface passivation on RET were studied by removing surface ligands through QD washing and adding an insulating ZnS shell. In addition, QD films were subjected to solid state ligand exchanges with thiolated ligands in order to mimic a layer-by-layer deposition method commonly used in the construction of QD photovoltaics. These solid state ligand exchanges exhibit drastic quenching of RET in the films. These experiments highlight the importance of understanding surface chemistry when designing photovoltaics that utilize RET.
499. Au–CsPbBr3 Hybrid Architecture: Anchoring Gold Nanoparticles on Cubic Perovskite Nanocrystals. Subila K. Balakrishnan and Prashant V. Kamat ACS Energy Lett. 2017, 2, pp 88–93
 A selective growth of gold (Au) nanoparticles on the corners of CsPbBr3 nanocrystals (NCs) is made possible with the treatment of Au(III) salts such as Au(III) bromide and Au(III) chloride in solution. The surface bound oleylamine ligands not only stabilize NCs but also facilitate reduction of the Au(III) salts followed by nucleation of the Au nanoparticles on the corners of the perovskite NCs. The luminescence quantum yield of NCs is decreased when Au nanoparticles are formed on the corners of CsPbBr3 NCs, suggesting interaction between the two systems. Formation of Au nanoparticles as well as an anion exchange is seen when Au(III) bromide was replaced with Au(III) chloride as a precursor. This simple strategy of designing perovskite–gold hybrid nanostructures with good colloidal stability offers new opportunities to explore their photocatalytic properties.
A selective growth of gold (Au) nanoparticles on the corners of CsPbBr3 nanocrystals (NCs) is made possible with the treatment of Au(III) salts such as Au(III) bromide and Au(III) chloride in solution. The surface bound oleylamine ligands not only stabilize NCs but also facilitate reduction of the Au(III) salts followed by nucleation of the Au nanoparticles on the corners of the perovskite NCs. The luminescence quantum yield of NCs is decreased when Au nanoparticles are formed on the corners of CsPbBr3 NCs, suggesting interaction between the two systems. Formation of Au nanoparticles as well as an anion exchange is seen when Au(III) bromide was replaced with Au(III) chloride as a precursor. This simple strategy of designing perovskite–gold hybrid nanostructures with good colloidal stability offers new opportunities to explore their photocatalytic properties.
2016
498. Band Diagram and Effects of the KSCN Treatment in TiO2/Sb2S3/CuSCN ETA Cells. Yafit Itzhaik, Tatyana Bendikov, Douglas Hines, Prashant V. Kamat, Hagai Cohen, and Gary Hodes J. Phys. Chem. C. 2016, 120 (1), pp 31–41
 Thiocyanate ion treatment, usually either LiSCN or KSCN, of the absorbing semiconductor before deposition of a CuSCN hole conducting layer is known to improve the performance of extremely thin absorber (ETA) solar cells by reducing the cell resistivity. However, in spite of several hypotheses, the mechanism behind this treatment outcome remains elusive. In this study, the interface between Sb2S3 and CuSCN in an ETA cell is investigated with surface spectroscopy and transient absorption spectroscopy to establish the mechanistic aspects of the KSCN treatment and the role it plays in improving the photovoltaic performance. The prominent factors that dictate the cell performance are (a) doping the interfacial CuSCN and thus preventing the formation of a sub-μm depleted layer and (b) passivating charge traps at the Sb2S(O)3 surface, which increases the rate of hole transfer from the absorber to the hole conductor. We further show that the treatment works just as well in improving photovoltaic performance when carried out after CuSCN deposition (post-treatment).
Thiocyanate ion treatment, usually either LiSCN or KSCN, of the absorbing semiconductor before deposition of a CuSCN hole conducting layer is known to improve the performance of extremely thin absorber (ETA) solar cells by reducing the cell resistivity. However, in spite of several hypotheses, the mechanism behind this treatment outcome remains elusive. In this study, the interface between Sb2S3 and CuSCN in an ETA cell is investigated with surface spectroscopy and transient absorption spectroscopy to establish the mechanistic aspects of the KSCN treatment and the role it plays in improving the photovoltaic performance. The prominent factors that dictate the cell performance are (a) doping the interfacial CuSCN and thus preventing the formation of a sub-μm depleted layer and (b) passivating charge traps at the Sb2S(O)3 surface, which increases the rate of hole transfer from the absorber to the hole conductor. We further show that the treatment works just as well in improving photovoltaic performance when carried out after CuSCN deposition (post-treatment).
497. Direct Observation of Reversible Transformation of CH3NH3PbI3 and NH4PbI3 Induced by Polar Gaseous Molecules. Weixin Huang, Joseph S. Manser, Subha Sadhu, Prashant V. Kamat and Sylwia Ptasinska J. Phys. Chem. Lett. 2016, 7 (24), pp 5068–5073
 Despite its competitive photovoltaic efficiency, the structural transformations of the prototypical hybrid perovskite, methylammonium lead iodide, are facilitated by interactions with polar molecules. Changes in optical and electronic properties upon exposure to ammonia potentially can enable the use of hybrid perovskites in gas-sensing applications. We investigated the effects of ammonia on CH3NH3PbI3 by exposing perovskite films to a wide range of vapor pressures. Spectroscopic analyses indicated that ammonium cations replaced the methylammonium cations in the perovskite crystal, thereby resulting in the formation of NH4PbI3. The transformation of CH3NH3PbI3 to NH4PbI3 caused distinct changes in the morphology of the film and its crystalline structure; however, the introduction of CH3NH2 gas reversed these changes. An in-depth understanding of the reversible chemical and structural alterations resulting from exposure to polar molecules can advance the development of hybrid perovskite sensors and provide insight into mechanisms by which perovskites convert due to interactions with polar molecules.
Despite its competitive photovoltaic efficiency, the structural transformations of the prototypical hybrid perovskite, methylammonium lead iodide, are facilitated by interactions with polar molecules. Changes in optical and electronic properties upon exposure to ammonia potentially can enable the use of hybrid perovskites in gas-sensing applications. We investigated the effects of ammonia on CH3NH3PbI3 by exposing perovskite films to a wide range of vapor pressures. Spectroscopic analyses indicated that ammonium cations replaced the methylammonium cations in the perovskite crystal, thereby resulting in the formation of NH4PbI3. The transformation of CH3NH3PbI3 to NH4PbI3 caused distinct changes in the morphology of the film and its crystalline structure; however, the introduction of CH3NH2 gas reversed these changes. An in-depth understanding of the reversible chemical and structural alterations resulting from exposure to polar molecules can advance the development of hybrid perovskite sensors and provide insight into mechanisms by which perovskites convert due to interactions with polar molecules.
496. Electrocatalytic Sensing with Reduced Graphene Oxide: Electron Shuttling between Redox Couples Anchored on a 2-D Surface. Victoria L. Bridewell, Christopher J. Karwacki and Prashant V. Kamat ACS Sens. 2016, 1 (10), pp 1203–1207
 The electron storage and shuttling capabilities of reduced graphene oxide (RGO) have been explored by anchoring two redox couples, methyl viologen (MV2+) and ferrocene (Fc). When an RGO modified glassy carbon electrode (GCE/RGO) was subjected to a cathodic scan, a quasi-reversible reduction of MV2+ was seen indicating a loss of electrons contributed to “charging” of RGO. These stored electrons can then be transported to oxidized Fc during the anodic scan through the C–C network of RGO. The recycling and peak current magnitude of oxidized and reduced forms of Fc during the anodic scan is strongly dependent on the scan rate, concentration, and extent of MV2+ reduction, either complete or partial, during the cathodic scan. This electrocatalytic property of RGO film enables the design of sensors and catalysts with the capacity to capture, store, and shuttle electrons and corroborate a boost in sensitivity for the electrochemical detection and conversion of low level analytes.
The electron storage and shuttling capabilities of reduced graphene oxide (RGO) have been explored by anchoring two redox couples, methyl viologen (MV2+) and ferrocene (Fc). When an RGO modified glassy carbon electrode (GCE/RGO) was subjected to a cathodic scan, a quasi-reversible reduction of MV2+ was seen indicating a loss of electrons contributed to “charging” of RGO. These stored electrons can then be transported to oxidized Fc during the anodic scan through the C–C network of RGO. The recycling and peak current magnitude of oxidized and reduced forms of Fc during the anodic scan is strongly dependent on the scan rate, concentration, and extent of MV2+ reduction, either complete or partial, during the cathodic scan. This electrocatalytic property of RGO film enables the design of sensors and catalysts with the capacity to capture, store, and shuttle electrons and corroborate a boost in sensitivity for the electrochemical detection and conversion of low level analytes.
495. Structural Phase- and Degradation-Dependent Thermal Conductivity of CH3NH3PbI3 Perovskite Thin Films Complexes. Zhi Guo, Seog Joon Yoon, Joseph S. Manser, Prashant V. Kamat and Tengfei Luo J. Phys. Chem. C. 2016, 120 (12), pp 6394–6401
 The optoelectronic properties of hybrid perovskites are a strong function of their physical structure, and understanding the fundamental steps involved in the formation of these films can aid in the optimization and rational design of devices with tailored properties. Here we investigate the structural and optical characteristics of CH3NH3PbI3 films prepared from solutions composed of stoichiometric and nonstoichiometric quantities of lead iodide and methylammonium iodide precursors. In the presence of excess organohalide salt, a precursor phase composed of various iodoplumbate complexes is stabilized. The complexes dominate the optical properties of as-deposited films. Upon thermal treatment, the iodoplumbate precursor phase gradually evolves into the final tetragonal perovskite structure. Employing transient absorption spectroscopy, we have succeeded in tracking this transformation and gain insight into the interplay between the solid-state precursor and perovskite phases at various stages of formation. Correlation between time-resolved spectroscopic data and structural character can aid in better defining the structure−property relationship of hybrid perovskite thin films.
The optoelectronic properties of hybrid perovskites are a strong function of their physical structure, and understanding the fundamental steps involved in the formation of these films can aid in the optimization and rational design of devices with tailored properties. Here we investigate the structural and optical characteristics of CH3NH3PbI3 films prepared from solutions composed of stoichiometric and nonstoichiometric quantities of lead iodide and methylammonium iodide precursors. In the presence of excess organohalide salt, a precursor phase composed of various iodoplumbate complexes is stabilized. The complexes dominate the optical properties of as-deposited films. Upon thermal treatment, the iodoplumbate precursor phase gradually evolves into the final tetragonal perovskite structure. Employing transient absorption spectroscopy, we have succeeded in tracking this transformation and gain insight into the interplay between the solid-state precursor and perovskite phases at various stages of formation. Correlation between time-resolved spectroscopic data and structural character can aid in better defining the structure−property relationship of hybrid perovskite thin films.
494. Transformation of Sintered CsPbBr3 Nanocrystals to Cubic CsPbI3 and Gradient CsPbBrxI3-x through Halide Exchange Hoffman, J. B.; Schleper, A. L.; Kamat, P. V. J. Am. Chem. Soc. 2016, 138 (27) pp 8603-8611
 All-inorganic cesium lead halide (CsPbX3, X = Br-, I-) perovskites could potentially provide comparable photovoltaic performance with enhanced stability compared to organic-inorganic lead halide species. However, small-bandgap cubic CsPbI3 has been difficult to study due to challenges forming CsPbI3 in the cubic phase. Here, a low-temperature procedure to form cubic CsPbI3 has been developed through a halide exchange reaction using films of sintered CsPbBr3 nanocrystals. The reaction was found to be strongly dependent upon temperature, featuring an Arrhenius relationship. Additionally, film thickness played a significant role in determining internal film structure at intermediate reaction times. Thin films (50 nm) showed only a small distribution of CsPbBrxI3-x species, while thicker films (350 nm) exhibited much broader distributions. Furthermore, internal film structure was ordered, featuring a compositional gradient within film. Transient absorption spectroscopy showed the influence of halide exchange on the excited state of the material. In thicker films, charge carriers were rapidly transferred to iodide-rich regions near the film surface within the first several picoseconds after excitation. This ultrafast vectorial charge-transfer process illustrates the potential of utilizing compositional gradients to direct charge flow in perovskite-based photovoltaics.
All-inorganic cesium lead halide (CsPbX3, X = Br-, I-) perovskites could potentially provide comparable photovoltaic performance with enhanced stability compared to organic-inorganic lead halide species. However, small-bandgap cubic CsPbI3 has been difficult to study due to challenges forming CsPbI3 in the cubic phase. Here, a low-temperature procedure to form cubic CsPbI3 has been developed through a halide exchange reaction using films of sintered CsPbBr3 nanocrystals. The reaction was found to be strongly dependent upon temperature, featuring an Arrhenius relationship. Additionally, film thickness played a significant role in determining internal film structure at intermediate reaction times. Thin films (50 nm) showed only a small distribution of CsPbBrxI3-x species, while thicker films (350 nm) exhibited much broader distributions. Furthermore, internal film structure was ordered, featuring a compositional gradient within film. Transient absorption spectroscopy showed the influence of halide exchange on the excited state of the material. In thicker films, charge carriers were rapidly transferred to iodide-rich regions near the film surface within the first several picoseconds after excitation. This ultrafast vectorial charge-transfer process illustrates the potential of utilizing compositional gradients to direct charge flow in perovskite-based photovoltaics.
493. Tracking Iodide and Bromide Ion Segregation in Mixed Halide Lead Perovskites during Photoirradiation Yoon, S. J.; Draguta, S.; Manser, J. S.; Sharia, O.; Schneider, W. F.; Kuno, M.; Kamat, P. V. ACS Energy Lett. 2016, 1 (1) pp 290-296
 Mixed halide lead perovskites (e.g., CH3NH3PbBrxI3-x) undergo phase segregation creating iodide-rich and bromide-rich domains when subjected to visible irradiation. This intriguing aspect of halide ion movement in mixed halide films is now being tracked through excited-state behavior using emission and transient absorption spectroscopy tools. These transient experiments have allowed us to establish the time scale with which such separation occurs under laser irradiation (405 nm, 25 mW/cm2 to 1.7 W/cm2) as well as dark recovery. While the phase separation occurs with a rate constant of 0.1-0.3 s-1, the recovery occurs over a time period of several minutes to an hour. The relative photoluminescence quantum yield observed for Br-rich regions (em. max 530 nm) is nearly 2 orders of magnitude lower than that of I-rich regions (em. max 760 nm) and arises from the fact that I-rich regions serve as sinks for photogenerated charge carriers. Understanding such cascading charge transfer to localized sites could further enable the design of gradient halide structures in mixed halide systems.
Mixed halide lead perovskites (e.g., CH3NH3PbBrxI3-x) undergo phase segregation creating iodide-rich and bromide-rich domains when subjected to visible irradiation. This intriguing aspect of halide ion movement in mixed halide films is now being tracked through excited-state behavior using emission and transient absorption spectroscopy tools. These transient experiments have allowed us to establish the time scale with which such separation occurs under laser irradiation (405 nm, 25 mW/cm2 to 1.7 W/cm2) as well as dark recovery. While the phase separation occurs with a rate constant of 0.1-0.3 s-1, the recovery occurs over a time period of several minutes to an hour. The relative photoluminescence quantum yield observed for Br-rich regions (em. max 530 nm) is nearly 2 orders of magnitude lower than that of I-rich regions (em. max 760 nm) and arises from the fact that I-rich regions serve as sinks for photogenerated charge carriers. Understanding such cascading charge transfer to localized sites could further enable the design of gradient halide structures in mixed halide systems.
492. Intriguing Optoelectronic Properties of Metal Halide Perovskites (Review) Manser, J. S.; Christians, J. A.; Kamat, P. V. Chem. Rev. 2016, 116 (21), pp 12956–13008
 A new chapter in the long and distinguished history of perovskites is being written with the breakthrough success of metal halide perovskites (MHPs) as solution-processed photovoltaic (PV) absorbers. The current surge in MHP research has largely arisen out of their rapid progress in PV devices; however, these materials are potentially suitable for a diverse array of optoelectronic applications. Like oxide perovskites, MHPs have ABX3 stoichiometry, where A and B are cations and X is a halide anion. Here, the underlying physical and photophysical properties of inorganic (A = inorganic) and hybrid organic-inorganic (A = organic) MHPs are reviewed with an eye toward their potential application in emerging optoelectronic technologies. Significant attention is given to the prototypical compound methylammonium lead iodide (CH3NH3PbI3) due to the preponderance of experimental and theoretical studies surrounding this material. We also discuss other salient MHP systems, including 2-dimensional compounds, where relevant. More specifically, this review is a critical account of the interrelation between MHP electronic structure, absorption, emission, carrier dynamics and transport, and other relevant photophysical processes that have propelled these materials to the forefront of modern optoelectronics research.
A new chapter in the long and distinguished history of perovskites is being written with the breakthrough success of metal halide perovskites (MHPs) as solution-processed photovoltaic (PV) absorbers. The current surge in MHP research has largely arisen out of their rapid progress in PV devices; however, these materials are potentially suitable for a diverse array of optoelectronic applications. Like oxide perovskites, MHPs have ABX3 stoichiometry, where A and B are cations and X is a halide anion. Here, the underlying physical and photophysical properties of inorganic (A = inorganic) and hybrid organic-inorganic (A = organic) MHPs are reviewed with an eye toward their potential application in emerging optoelectronic technologies. Significant attention is given to the prototypical compound methylammonium lead iodide (CH3NH3PbI3) due to the preponderance of experimental and theoretical studies surrounding this material. We also discuss other salient MHP systems, including 2-dimensional compounds, where relevant. More specifically, this review is a critical account of the interrelation between MHP electronic structure, absorption, emission, carrier dynamics and transport, and other relevant photophysical processes that have propelled these materials to the forefront of modern optoelectronics research.
491. Structural Phase- and Degradation-Dependent Thermal Conductivity of CH3NH3PbI3 Perovskite Thin Films Guo, Z.; Yoon, S. J.; Manser, J. S.; Kamat, P. V.; Luo, T. J. Phys. Chem. C 2016, 120 (2) pp 6394-6401
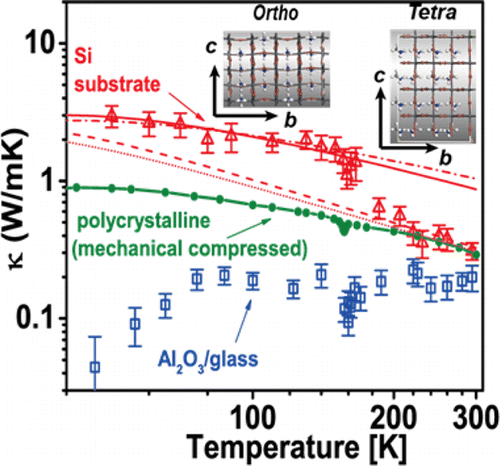 Organic-inorganic lead halide perovskites have shown great promise in photovoltaics and optoelectronics. In these applications, device performance and reliability can be strongly influenced by thermal transport in the materials. Through laser pump-probe experiments, different microstructures of CH3NH3PbI3 perovskite thin films are found to give rise to different phonon scattering mechanism. The thermal conductivity in CH3NH3PbI3 neat film decreases with temperature. Even though this agrees with the behavior of its bulk crystalline counterparts, an apparent thermal conductivity change near the structural phase transition temperature of this perovskite (orthorhombic vs tetragonal) has only been observed in the spin-coated films. Analyses suggest that this may be attributed to either an energy landscape change related to organic cation disorder or the thickness change of ferroelectric domain walls formed in the neat perovskite films that affects the phonon scattering at the domain boundaries. In contrast, no thermal conductivity discontinuity has been observed in the CH3NH3PbI3 /Al2O3 mesostructured films, where the thermal conductivity first shows an increasing trend at low temperature (<80 K) and then stays nearly constant. Such a trend is typical in amorphous materials and nanostructured composites where phonon scatterings are due to morphological disorder and internal interfaces play key roles in the thermal transport. When exposed to the ambient environment, humidity induced degradation is found to have a significant impact on the overall thermal conductivity of the spin-coated CH3NH3PbI3 neat film.
Organic-inorganic lead halide perovskites have shown great promise in photovoltaics and optoelectronics. In these applications, device performance and reliability can be strongly influenced by thermal transport in the materials. Through laser pump-probe experiments, different microstructures of CH3NH3PbI3 perovskite thin films are found to give rise to different phonon scattering mechanism. The thermal conductivity in CH3NH3PbI3 neat film decreases with temperature. Even though this agrees with the behavior of its bulk crystalline counterparts, an apparent thermal conductivity change near the structural phase transition temperature of this perovskite (orthorhombic vs tetragonal) has only been observed in the spin-coated films. Analyses suggest that this may be attributed to either an energy landscape change related to organic cation disorder or the thickness change of ferroelectric domain walls formed in the neat perovskite films that affects the phonon scattering at the domain boundaries. In contrast, no thermal conductivity discontinuity has been observed in the CH3NH3PbI3 /Al2O3 mesostructured films, where the thermal conductivity first shows an increasing trend at low temperature (<80 K) and then stays nearly constant. Such a trend is typical in amorphous materials and nanostructured composites where phonon scatterings are due to morphological disorder and internal interfaces play key roles in the thermal transport. When exposed to the ambient environment, humidity induced degradation is found to have a significant impact on the overall thermal conductivity of the spin-coated CH3NH3PbI3 neat film.
490. Band Diagram and Effects of the KSCN Treatment in TiO2/Sb2S3/CuSCN ETA Cells Itzhaik, Y.; Bendikov, T.; Hines, D.; Kamat, P. V.; Cohen, H.; Hodes, G. J. Phys. Chem. C 2016, 120 (1) pp 31-41
 Thiocyanate ion treatment, usually either LiSCN or KSCN, of the absorbing semiconductor before deposition of a CuSCN hole conducting layer is known to improve the performance of extremely thin absorber (ETA) solar cells by reducing the cell resistivity. However, in spite of several hypotheses, the mechanism behind this treatment outcome remains elusive. In this study, the interface between Sb2S3 and CuSCN in an ETA cell is investigated with surface spectroscopy and transient absorption spectroscopy to establish the mechanistic aspects of the KSCN treatment and the role it plays in improving the photovoltaic performance. The prominent factors that dictate the cell performance are (a) doping the interfacial CuSCN and thus preventing the formation of a sub-μm depleted layer and (b) passivating charge traps at the Sb2S(O)3 surface, which increases the rate of hole transfer from the absorber to the hole conductor. We further show that the treatment works just as well in improving photovoltaic performance when carried out after CuSCN deposition (post-treatment).
Thiocyanate ion treatment, usually either LiSCN or KSCN, of the absorbing semiconductor before deposition of a CuSCN hole conducting layer is known to improve the performance of extremely thin absorber (ETA) solar cells by reducing the cell resistivity. However, in spite of several hypotheses, the mechanism behind this treatment outcome remains elusive. In this study, the interface between Sb2S3 and CuSCN in an ETA cell is investigated with surface spectroscopy and transient absorption spectroscopy to establish the mechanistic aspects of the KSCN treatment and the role it plays in improving the photovoltaic performance. The prominent factors that dictate the cell performance are (a) doping the interfacial CuSCN and thus preventing the formation of a sub-μm depleted layer and (b) passivating charge traps at the Sb2S(O)3 surface, which increases the rate of hole transfer from the absorber to the hole conductor. We further show that the treatment works just as well in improving photovoltaic performance when carried out after CuSCN deposition (post-treatment).
489. Making and Breaking of Lead Halide Perovskites Manser, J. S.; Saidaminov, M. I.; Christians, J. A.; Bakr, O. M.; Kamat, P. V. Acc. Chem. Res. 2016, 49 (2) pp 330-338
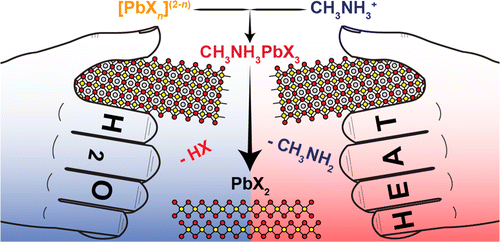 A new front-runner has emerged in the field of next-generation photovoltaics. A unique class of materials, known as organic metal halide perovskites, bridges the gap between low-cost fabrication and exceptional device performance. These compounds can be processed at low temperature (typically in the range 80-150 oC) and readily self-assemble from the solution phase into high-quality semiconductor thin films. The low energetic barrier for crystal formation has mixed consequences. On one hand, it enables inexpensive processing and both optical and electronic tunability. The caveat, however, is that many as-formed lead halide perovskite thin films lack chemical and structural stability, undergoing rapid degradation in the presence of moisture or heat. To date, improvements in perovskite solar cell efficiency have resulted primarily from better control over thin film morphology, manipulation of the stoichiometry and chemistry of lead halide and alkylammonium halide precursors, and the choice of solvent treatment. Proper characterization and tuning of processing parameters can aid in rational optimization of perovskite devices. Likewise, gaining a comprehensive understanding of the degradation mechanism and identifying components of the perovskite structure that may be particularly susceptible to attack by moisture are vital to mitigate device degradation under operating conditions.
This Account provides insight into the lifecycle of organic-inorganic lead halide perovskites, including (i) the nature of the precursor solution, (ii) formation of solid-state perovskite thin films and single crystals, and (iii) transformation of perovskites into hydrated phases upon exposure to moisture. In particular, spectroscopic and structural characterization techniques shed light on the thermally driven evolution of the perovskite structure. By tuning precursor stoichiometry and chemistry, and thus the lead halide charge-transfer complexes present in solution, crystallization kinetics can be tailored to yield improved thin film homogeneity. Because degradation of the as-formed perovskite film is in many ways analogous to its initial formation, the same suite of monitoring techniques reveals the moisture-induced transformation of low band gap methylammonium lead iodide (CH3NH3PbI3) to wide band gap hydrate compounds. The rate of degradation is increased upon exposure to light. Interestingly, the hydration process is reversible under certain conditions. This facile formation and subsequent chemical lability raises the question of whether CH3NH3PbI3 and its analogues are thermodynamically stable phases, thus posing a significant challenge to the development of transformative perovskite photovoltaics. Adequately addressing issues of structural and chemical stability under real-world operating conditions is paramount if perovskite solar cells are to make an impact beyond the benchtop. Expanding our fundamental knowledge of lead halide perovskite formation and degradation pathways can facilitate fabrication of stable, high-quality perovskite thin films for the next generation of photovoltaic and light emitting devices.
A new front-runner has emerged in the field of next-generation photovoltaics. A unique class of materials, known as organic metal halide perovskites, bridges the gap between low-cost fabrication and exceptional device performance. These compounds can be processed at low temperature (typically in the range 80-150 oC) and readily self-assemble from the solution phase into high-quality semiconductor thin films. The low energetic barrier for crystal formation has mixed consequences. On one hand, it enables inexpensive processing and both optical and electronic tunability. The caveat, however, is that many as-formed lead halide perovskite thin films lack chemical and structural stability, undergoing rapid degradation in the presence of moisture or heat. To date, improvements in perovskite solar cell efficiency have resulted primarily from better control over thin film morphology, manipulation of the stoichiometry and chemistry of lead halide and alkylammonium halide precursors, and the choice of solvent treatment. Proper characterization and tuning of processing parameters can aid in rational optimization of perovskite devices. Likewise, gaining a comprehensive understanding of the degradation mechanism and identifying components of the perovskite structure that may be particularly susceptible to attack by moisture are vital to mitigate device degradation under operating conditions.
This Account provides insight into the lifecycle of organic-inorganic lead halide perovskites, including (i) the nature of the precursor solution, (ii) formation of solid-state perovskite thin films and single crystals, and (iii) transformation of perovskites into hydrated phases upon exposure to moisture. In particular, spectroscopic and structural characterization techniques shed light on the thermally driven evolution of the perovskite structure. By tuning precursor stoichiometry and chemistry, and thus the lead halide charge-transfer complexes present in solution, crystallization kinetics can be tailored to yield improved thin film homogeneity. Because degradation of the as-formed perovskite film is in many ways analogous to its initial formation, the same suite of monitoring techniques reveals the moisture-induced transformation of low band gap methylammonium lead iodide (CH3NH3PbI3) to wide band gap hydrate compounds. The rate of degradation is increased upon exposure to light. Interestingly, the hydration process is reversible under certain conditions. This facile formation and subsequent chemical lability raises the question of whether CH3NH3PbI3 and its analogues are thermodynamically stable phases, thus posing a significant challenge to the development of transformative perovskite photovoltaics. Adequately addressing issues of structural and chemical stability under real-world operating conditions is paramount if perovskite solar cells are to make an impact beyond the benchtop. Expanding our fundamental knowledge of lead halide perovskite formation and degradation pathways can facilitate fabrication of stable, high-quality perovskite thin films for the next generation of photovoltaic and light emitting devices.
488. Two Distinct Transitions in CuxInS2 Quantum Dots. Bandgap versus Sub-Bandgap Excitations in Copper-Deficient Structures Jara, D. H.; Stamplecoskie, K. G.; Kamat, P. V. J. Phys. Chem. Lett. 2016, 7 pp 1452-1459
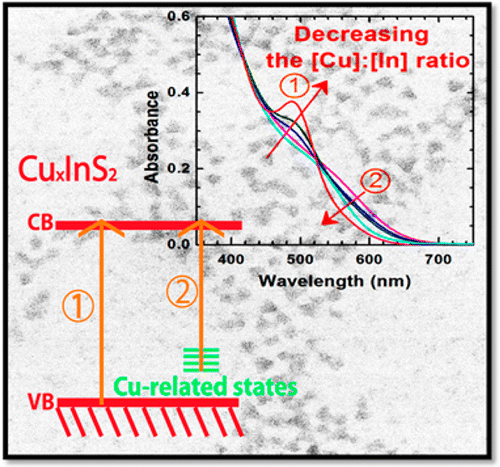 Cu-deficient CuInS2 quantum dots (QDs) synthesized by varying the [Cu]:[In] ratio allow modulation of optical properties as well as identification of the radiative emission pathways. Absorption and emission spectral features showed a strong dependence on the [Cu]:[In] ratio of CuxInS2 QDs, indicating two independent optical transitions. These effects are pronounced in transient absorption spectra. The bleaching of band edge absorption and broad tail absorption bands in the subpicosecond-nanosecond time scale provide further evidence for the dual optical transitions. The recombination process as monitored by photoemission decay indicated the involvement of surface traps in addition to the bandgap and sub-bandgap transitions. Better understanding of the origin of the optical transitions and their influence on the photodynamics will enable utilization of ternary semiconductor quantum dots in display and photovoltaic devices.
Cu-deficient CuInS2 quantum dots (QDs) synthesized by varying the [Cu]:[In] ratio allow modulation of optical properties as well as identification of the radiative emission pathways. Absorption and emission spectral features showed a strong dependence on the [Cu]:[In] ratio of CuxInS2 QDs, indicating two independent optical transitions. These effects are pronounced in transient absorption spectra. The bleaching of band edge absorption and broad tail absorption bands in the subpicosecond-nanosecond time scale provide further evidence for the dual optical transitions. The recombination process as monitored by photoemission decay indicated the involvement of surface traps in addition to the bandgap and sub-bandgap transitions. Better understanding of the origin of the optical transitions and their influence on the photodynamics will enable utilization of ternary semiconductor quantum dots in display and photovoltaic devices.
487. How Lead Halide Complex Chemistry Dictates the Composition of Mixed Halide Perovskites Yoon, S. J.; Stamplecoskie, K. G.; Kamat, P. V. J. Phys. Chem. Lett. 2016, 7 pp 1368-1373
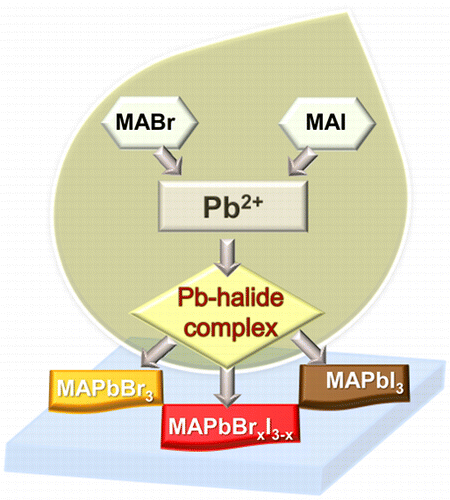 Varying the halide ratio (e.g., Br-:I-) is a convenient approach to tune the bandgap of organic lead halide perovskites. The complexation between Pb2+ and halide ions is the primary step in dictating the overall composition, and optical properties of the annealed perovskite structure. The complexation between Pb2+ and Br- is nearly 7 times greater than the complexation between Pb2+ and I-), thus making Br- a dominant binding species in mixed halide systems. Emission and transient absorption measurements show a strong dependence of excited state behavior on the composition of halide ions employed in the precursor solution. When excess halide (X = Br- and I-) are present in the precursor solution (0.3 M PbX2 and 0.9 M CH3NH3X), the exclusive binding of Pb2+ with Br- results in the formation of CH3NH3PbBr3 perovskites as opposed to mixed halide perovskite.
Varying the halide ratio (e.g., Br-:I-) is a convenient approach to tune the bandgap of organic lead halide perovskites. The complexation between Pb2+ and halide ions is the primary step in dictating the overall composition, and optical properties of the annealed perovskite structure. The complexation between Pb2+ and Br- is nearly 7 times greater than the complexation between Pb2+ and I-), thus making Br- a dominant binding species in mixed halide systems. Emission and transient absorption measurements show a strong dependence of excited state behavior on the composition of halide ions employed in the precursor solution. When excess halide (X = Br- and I-) are present in the precursor solution (0.3 M PbX2 and 0.9 M CH3NH3X), the exclusive binding of Pb2+ with Br- results in the formation of CH3NH3PbBr3 perovskites as opposed to mixed halide perovskite.
486. Modulation of Cu2-xS Nanocrystal Plasmon Resonance through Reversible Photoinduced Electron Transfer Alam, R.; Labine, M.; Karwacki, C. J.; Kamat, P. V. ACS Nano 2016, 10 (2) pp 2880-2886
 Copper sulfide (Cu2-xS) nanocrystals with nonstoichiometric composition exhibit plasmon resonance in the near-infrared region. Compositional changes and varying electron density markedly affect the position and intensity of the plasmon resonance. We report a photochemically induced phenomenon of modulating the plasmon resonance in a controlled fashion. As photogenerated reduced methyl viologen radicals transfer electrons to Cu2-xS in inert solutions, we observe a decrease in localized surface plasmon resonance (LSPR) absorbance at 1160 nm. Upon exposure to air, the plasmon resonance band recovers as stored electrons are scavenged away by oxygen. This cycle of electron charge and discharge of Cu2-xS nanocrystals is reversible and can be repeated through photoirradiation in N2 saturated solution followed by exposure of the suspension to air. The spectroscopic studies that provide mechanistic insights into the reversible charging and discharging of plasmonic Cu2-xS are discussed.
Copper sulfide (Cu2-xS) nanocrystals with nonstoichiometric composition exhibit plasmon resonance in the near-infrared region. Compositional changes and varying electron density markedly affect the position and intensity of the plasmon resonance. We report a photochemically induced phenomenon of modulating the plasmon resonance in a controlled fashion. As photogenerated reduced methyl viologen radicals transfer electrons to Cu2-xS in inert solutions, we observe a decrease in localized surface plasmon resonance (LSPR) absorbance at 1160 nm. Upon exposure to air, the plasmon resonance band recovers as stored electrons are scavenged away by oxygen. This cycle of electron charge and discharge of Cu2-xS nanocrystals is reversible and can be repeated through photoirradiation in N2 saturated solution followed by exposure of the suspension to air. The spectroscopic studies that provide mechanistic insights into the reversible charging and discharging of plasmonic Cu2-xS are discussed.
485. Spatially Non-uniform Trap State Densities in Solution-Processed Hybrid Perovskite Thin Films Draguta, S.; Thakur, S.; Morozov, Y.; Wang, Y.; Manser, J. S.; Kamat, P. V.; Kuno, M. J. Phys. Chem. Lett. 2016, 7 (4) pp 715-721
 The facile solution-processability of methylammonium lead halide (CH3NH3PbI3) perovskites has catalyzed the development of inexpensive, hybrid perovskite-based optoelectronics. It is apparent, though, that solution-processed CH3NH3PbI3 films possess local emission heterogeneities, stemming from electronic disorder in the material. Herein we investigate the spatially resolved emission properties of CH3NH3PbI3 thin films through detailed emission intensity versus excitation intensity measurements. These studies enable us to establish the existence of nonuniform trap density variations wherein regions of CH3NH3PbI3 films exhibit effective free carrier recombination while others exhibit emission dynamics strongly influenced by the presence of trap states. Such trap density variations lead to spatially varying emission quantum yields and correspondingly impact the performance of both methylammonium lead halide perovskite solar cells and other hybrid perovskite-based devices. Of additional note is that the observed spatial extent of the optical disorder extends over length scales greater than that of underlying crystalline domains, suggesting the existence of other factors, beyond grain boundary-related nonradiative recombination channels, which lead to significant intrafilm optical heterogeneities.
The facile solution-processability of methylammonium lead halide (CH3NH3PbI3) perovskites has catalyzed the development of inexpensive, hybrid perovskite-based optoelectronics. It is apparent, though, that solution-processed CH3NH3PbI3 films possess local emission heterogeneities, stemming from electronic disorder in the material. Herein we investigate the spatially resolved emission properties of CH3NH3PbI3 thin films through detailed emission intensity versus excitation intensity measurements. These studies enable us to establish the existence of nonuniform trap density variations wherein regions of CH3NH3PbI3 films exhibit effective free carrier recombination while others exhibit emission dynamics strongly influenced by the presence of trap states. Such trap density variations lead to spatially varying emission quantum yields and correspondingly impact the performance of both methylammonium lead halide perovskite solar cells and other hybrid perovskite-based devices. Of additional note is that the observed spatial extent of the optical disorder extends over length scales greater than that of underlying crystalline domains, suggesting the existence of other factors, beyond grain boundary-related nonradiative recombination channels, which lead to significant intrafilm optical heterogeneities.
484. Evolution of Chemical Composition, Morphology, and Photovoltaic Efficiency of CH3NH3PbI3 Perovskite under Ambient Conditions Huang, W.; Manser, J. S.; Kamat, P. V.; Ptasinska, S. Chem. Mater. 2016, 28 (1) pp 303-311
 The surface composition and morphology of CH3NH3PbI3 perovskite films stored for several days under ambient conditions were investigated by X-ray photoelectron spectroscopy, scanning electron microscopy, and X-ray diffraction techniques. Chemical analysis revealed the loss of CH3NH3+ and I- species from CH3NH3PbI3 and its subsequent decomposition into lead carbonate, lead hydroxide, and lead oxide. After long-term storage under ambient conditions, morphological analysis revealed the transformation of randomly distributed defects and cracks, initially present in the densely packed crystalline structure, into relatively small grains. In contrast to PbI2 powder, CH3NH3PbI3 exhibited a different degradation trend under ambient conditions. Therefore, we propose a plausible CH3NH3PbI3 decomposition pathway that explains the changes in the chemical composition of CH3NH3PbI3 under ambient conditions. In addition, films stored under such conditions were incorporated into photovoltaic cells, and their performances were examined. The chemical changes in the decomposed films were found to cause a significant decrease in the photovoltaic efficiency of CH3NH3PbI3.
The surface composition and morphology of CH3NH3PbI3 perovskite films stored for several days under ambient conditions were investigated by X-ray photoelectron spectroscopy, scanning electron microscopy, and X-ray diffraction techniques. Chemical analysis revealed the loss of CH3NH3+ and I- species from CH3NH3PbI3 and its subsequent decomposition into lead carbonate, lead hydroxide, and lead oxide. After long-term storage under ambient conditions, morphological analysis revealed the transformation of randomly distributed defects and cracks, initially present in the densely packed crystalline structure, into relatively small grains. In contrast to PbI2 powder, CH3NH3PbI3 exhibited a different degradation trend under ambient conditions. Therefore, we propose a plausible CH3NH3PbI3 decomposition pathway that explains the changes in the chemical composition of CH3NH3PbI3 under ambient conditions. In addition, films stored under such conditions were incorporated into photovoltaic cells, and their performances were examined. The chemical changes in the decomposed films were found to cause a significant decrease in the photovoltaic efficiency of CH3NH3PbI3.
2015
483. Dynamics of Photogenerated Charge Carriers in WO3/BiVO4 Heterojunction Photoanodes Grigioni, I.; Stamplecoskie, K. G.; Selli, E.; Kamat, P. V. J. Phys. Chem. C 2015, 119 (36) pp 20792-20800
 Bismuth vanadate (BiVO4) with a band gap of ∼2.4 eV has emerged as one of the visible photocatalysts that can absorb light below 520 nm. The electron/hole pairs that are generated following BiVO4 band gap excitation are effective for water splitting, especially when BiVO4 is combined with other metal oxides such as WO3. We report a solution processed method for designing transparent WO3/BiVO4 heterojunction electrodes and observe a synergistic effect on the photoelectrochemical activity of WO3/BiVO4, with the combined system performing dramatically better than either individual component. Using ultrafast transient absorption spectroscopy, we elucidated the electronic interaction between WO3 and excited BiVO4. Moreover, the photocatalytic reduction of thionine by WO3/BiVO4 as well as by each individual oxide component is used to track electron injection processes and determine the energetics of the studied systems. In the composite WO3/BiVO4 film a shifted quasi-Fermi level results, due to electronic equilibration between the two materials. The better performance of WO3/BiVO4 heterojunction electrodes is thus a consequence of the electron injection from BiVO4 into WO3, followed by back electron transfer from WO3 to the holes in BiVO4.
Bismuth vanadate (BiVO4) with a band gap of ∼2.4 eV has emerged as one of the visible photocatalysts that can absorb light below 520 nm. The electron/hole pairs that are generated following BiVO4 band gap excitation are effective for water splitting, especially when BiVO4 is combined with other metal oxides such as WO3. We report a solution processed method for designing transparent WO3/BiVO4 heterojunction electrodes and observe a synergistic effect on the photoelectrochemical activity of WO3/BiVO4, with the combined system performing dramatically better than either individual component. Using ultrafast transient absorption spectroscopy, we elucidated the electronic interaction between WO3 and excited BiVO4. Moreover, the photocatalytic reduction of thionine by WO3/BiVO4 as well as by each individual oxide component is used to track electron injection processes and determine the energetics of the studied systems. In the composite WO3/BiVO4 film a shifted quasi-Fermi level results, due to electronic equilibration between the two materials. The better performance of WO3/BiVO4 heterojunction electrodes is thus a consequence of the electron injection from BiVO4 into WO3, followed by back electron transfer from WO3 to the holes in BiVO4.
482. Understanding the Implication of Carrier Diffusion Length in Photovoltaic Cells (Viewpoint) Hodes, G.; Kamat, P. V. J. Phys. Chem. Lett. 2015, 6 (20) pp 4090-4092
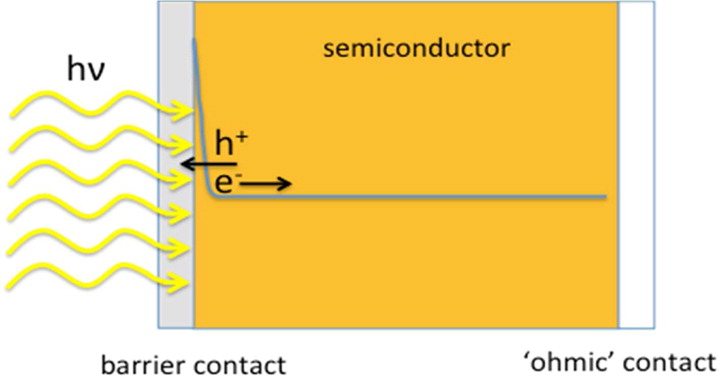 The purpose of this Viewpoint is to dispel a commonly held misconception when comparing diffusion lengths and discuss how variation in the measuring techniques can bring about differences in the measured values. The diffusion length, Ld, of electrons or holes in a semiconductor is defined by the average distance the relevant charge moves in the semiconductor. It is influenced by the average distance the relevant charge moves in the semiconductor (for example, in photovoltaic cells, which is the topic of interest in this Viewpoint) and recombination/extraction from the semiconductor.
The purpose of this Viewpoint is to dispel a commonly held misconception when comparing diffusion lengths and discuss how variation in the measuring techniques can bring about differences in the measured values. The diffusion length, Ld, of electrons or holes in a semiconductor is defined by the average distance the relevant charge moves in the semiconductor. It is influenced by the average distance the relevant charge moves in the semiconductor (for example, in photovoltaic cells, which is the topic of interest in this Viewpoint) and recombination/extraction from the semiconductor.
481. Evolution of Organic–Inorganic Lead Halide Perovskite from Solid-State Iodoplumbate Complexes Manser, J. S.; Reid, B.; Kamat, P. V. J. Phys. Chem C 2015, 119, 17065–17073
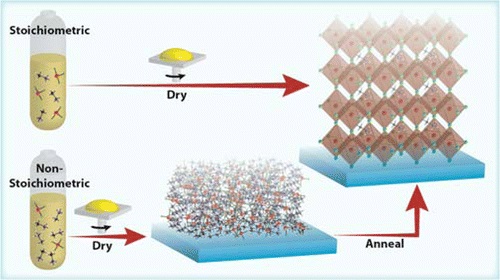 The optoelectronic properties of hybrid perovskites are a strong function of their physical structure, and understanding the fundamental steps involved in the formation of these films can aid in the optimization and rational design of devices with tailored properties. Here we investigate the structural and optical characteristics of CH3NH3PbI3 films prepared from solutions composed of stoichiometric and nonstoichiometric quantities of lead iodide and methylammonium iodide precursors. In the presence of excess organohalide salt, a precursor phase composed of various iodoplumbate complexes is stabilized. The complexes dominate the optical properties of as-deposited films. Upon thermal treatment, the iodoplumbate precursor phase gradually evolves into the final tetragonal perovskite structure. Employing transient absorption spectroscopy, we have succeeded in tracking this transformation and gain insight into the interplay between the solid-state precursor and perovskite phases at various stages of formation. Correlation between time-resolved spectroscopic data and structural character can aid in better defining the structure–property relationship of hybrid perovskite thin films.
The optoelectronic properties of hybrid perovskites are a strong function of their physical structure, and understanding the fundamental steps involved in the formation of these films can aid in the optimization and rational design of devices with tailored properties. Here we investigate the structural and optical characteristics of CH3NH3PbI3 films prepared from solutions composed of stoichiometric and nonstoichiometric quantities of lead iodide and methylammonium iodide precursors. In the presence of excess organohalide salt, a precursor phase composed of various iodoplumbate complexes is stabilized. The complexes dominate the optical properties of as-deposited films. Upon thermal treatment, the iodoplumbate precursor phase gradually evolves into the final tetragonal perovskite structure. Employing transient absorption spectroscopy, we have succeeded in tracking this transformation and gain insight into the interplay between the solid-state precursor and perovskite phases at various stages of formation. Correlation between time-resolved spectroscopic data and structural character can aid in better defining the structure–property relationship of hybrid perovskite thin films.
480. CdSe/CdS Nanorod Photocatalysts: Tuning the Interfacial Charge Transfer Process through Shell Length Bridewell, V. L.; Alam, R.; Karwacki, C. J.; Kamat, P. V. Chem. Mater. 2015, 27, 5064-5071
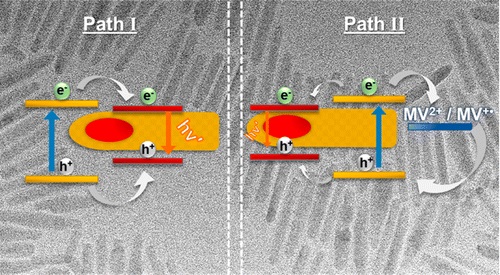 CdSe/CdS core/shell semiconductor nanorods (NR) with rod-in-rod morphology offer new strategies for designing highly emissive nanostructures. The interplay between energetically matched semiconductors results in enhanced emission from the CdSe core. In order to further evaluate the cooperative role of these two semiconductors in a core/shell geometry, we have probed the photoinduced charge transfer between CdSe/CdS core/shell semiconductor NR and methyl viologen (MV2+). The quenching of the emission by the electron acceptor, MV2+, as well as the production of electron transfer product MV•+ depends on the aspect ratio (l/w) of the NR thus pointing out the role of CdS shell in determining the overall photocatalytic efficiency. Transient absorption measurements show that the presence of MV2+ influences only the bleaching recovery of the CdS shell and not of the CdSe core recovery. Thus, optimization of shell aspect ratio plays a crucial role in maximizing the efficiency of this photocatalytic system.
CdSe/CdS core/shell semiconductor nanorods (NR) with rod-in-rod morphology offer new strategies for designing highly emissive nanostructures. The interplay between energetically matched semiconductors results in enhanced emission from the CdSe core. In order to further evaluate the cooperative role of these two semiconductors in a core/shell geometry, we have probed the photoinduced charge transfer between CdSe/CdS core/shell semiconductor NR and methyl viologen (MV2+). The quenching of the emission by the electron acceptor, MV2+, as well as the production of electron transfer product MV•+ depends on the aspect ratio (l/w) of the NR thus pointing out the role of CdS shell in determining the overall photocatalytic efficiency. Transient absorption measurements show that the presence of MV2+ influences only the bleaching recovery of the CdS shell and not of the CdSe core recovery. Thus, optimization of shell aspect ratio plays a crucial role in maximizing the efficiency of this photocatalytic system.
479. Spatial and temporal imaging of long-range charge transport in perovskite thin films by ultrafast microscopy Guo, Z.; Manser, J. S.; Wan, Y.; Kamat, P. V.; Huang, L. Nat. Commun. 2015, 6, Article No. 7471
 Charge carrier diffusion coefficient and length are important physical parameters for semiconducting materials. Long-range carrier diffusion in perovskite thin films has led to remarkable solar cell efficiencies; however, spatial and temporal mechanisms of charge transport remain unclear. Here we present a direct measurement of carrier transport in space and in time by mapping carrier density with simultaneous ultrafast time resolution and ~50-nm spatial precision in perovskite thin films using transient absorption microscopy. These results directly visualize long-range carrier transport of ~220 nm in 2 ns for solution-processed polycrystalline CH3NH3PbI3, thin films. Variations of the carrier diffusion coefficient at the μm length scale have been observed with values ranging between 0.05 and 0.08 cm2 s−1. The spatially and temporally resolved measurements reported here underscore the importance of the local morphology and establish an important first step towards discerning the underlying transport properties of perovskite materials.
Charge carrier diffusion coefficient and length are important physical parameters for semiconducting materials. Long-range carrier diffusion in perovskite thin films has led to remarkable solar cell efficiencies; however, spatial and temporal mechanisms of charge transport remain unclear. Here we present a direct measurement of carrier transport in space and in time by mapping carrier density with simultaneous ultrafast time resolution and ~50-nm spatial precision in perovskite thin films using transient absorption microscopy. These results directly visualize long-range carrier transport of ~220 nm in 2 ns for solution-processed polycrystalline CH3NH3PbI3, thin films. Variations of the carrier diffusion coefficient at the μm length scale have been observed with values ranging between 0.05 and 0.08 cm2 s−1. The spatially and temporally resolved measurements reported here underscore the importance of the local morphology and establish an important first step towards discerning the underlying transport properties of perovskite materials.
478. Multifaceted Excited State of CH3NH3PbI3. Charge Separation, Recombination, and Trapping Christians, J. A.; Manser, J. S.; Kamat, P. V. J. Phys. Chem. Lett. 2015, 6, 2086–2095.
 A need to understand the excited-state behavior of organic–inorganic hybrid perovskites, such as CH3NH3PbI3, has arisen due to the rapid development of perovskite solar cells. The photoinduced processes leading to the efficient charge separation observed in these materials remain somewhat elusive. This Perspective presents an overview of the initial attempts to characterize the excited-state and charge recombination dynamics in the prototypical material CH3NH3PbI3. While much has been accomplished in designing high-efficiency solar cells, the multifaceted nature of the CH3NH3PbI3 excited state offers ample challenges for the photovoltaic community to better comprehend. Building on this foundation may enable us to tackle the stability concerns that have shadowed the rise of perovskite solar cells. Furthermore, a better understanding of the excited-state properties can provide insight into the specific properties that have thrust this material to the forefront of photovoltaic research.
A need to understand the excited-state behavior of organic–inorganic hybrid perovskites, such as CH3NH3PbI3, has arisen due to the rapid development of perovskite solar cells. The photoinduced processes leading to the efficient charge separation observed in these materials remain somewhat elusive. This Perspective presents an overview of the initial attempts to characterize the excited-state and charge recombination dynamics in the prototypical material CH3NH3PbI3. While much has been accomplished in designing high-efficiency solar cells, the multifaceted nature of the CH3NH3PbI3 excited state offers ample challenges for the photovoltaic community to better comprehend. Building on this foundation may enable us to tackle the stability concerns that have shadowed the rise of perovskite solar cells. Furthermore, a better understanding of the excited-state properties can provide insight into the specific properties that have thrust this material to the forefront of photovoltaic research.
477. Synergistic Effects in the Coupling of Plasmon Resonance of Metal Nanoparticles with Excited Gold Clusters Stamplecoskie, K. G.; Kamat, P. V. J. Phys. Chem. Lett. 2015, 6 (5), 1870–1875.
 When molecules or clusters are within the proximity of metal particles, their electronic transitions can be drastically enhanced. We have now probed the off-resonance excitation of molecule-like, glutathione-capped gold clusters (Au-GSH) in the close proximity of larger (plasmonic) Au and Ag nanoparticles. The excited state absorption spectrum of Au-GSH* is obtained with monophotonic excitation. The characteristic absorption of Au-GSH* allows us to probe the influence of excited plasmonic nanoparticles coupled with the clusters. Although infrared (775 nm) lasers pulses do not produce Au-GSH*, the excited states of these clusters are formed when coupled with metal (Au, Ag) nanoparticles. Interestingly, the coupled excitation of Au-GSH/AgNP with 775 nm laser pulses also results in an enhanced field effect, as seen from increased plasmon response of the metal nanoparticles. Transient absorption measurements confirm the synergy between these two inherently different nanomaterials, causing them to display greater excitation features. Better understanding of metal cluster–metal nanoparticle interactions will have important implications in designing light harvesting systems, and optoelectronic devices.
When molecules or clusters are within the proximity of metal particles, their electronic transitions can be drastically enhanced. We have now probed the off-resonance excitation of molecule-like, glutathione-capped gold clusters (Au-GSH) in the close proximity of larger (plasmonic) Au and Ag nanoparticles. The excited state absorption spectrum of Au-GSH* is obtained with monophotonic excitation. The characteristic absorption of Au-GSH* allows us to probe the influence of excited plasmonic nanoparticles coupled with the clusters. Although infrared (775 nm) lasers pulses do not produce Au-GSH*, the excited states of these clusters are formed when coupled with metal (Au, Ag) nanoparticles. Interestingly, the coupled excitation of Au-GSH/AgNP with 775 nm laser pulses also results in an enhanced field effect, as seen from increased plasmon response of the metal nanoparticles. Transient absorption measurements confirm the synergy between these two inherently different nanomaterials, causing them to display greater excitation features. Better understanding of metal cluster–metal nanoparticle interactions will have important implications in designing light harvesting systems, and optoelectronic devices.
476. Best Practices in Perovskite Solar Cell Efficiency Measurements. Avoiding the Error of Making Bad Cells Look Good (Viewpoint) Christians, J. A.; Manser, J. S.; Kamat, P. V. J. Phys. Chem. Lett. 2015, 6 (5), 852–857.
 Perovskite solar cells employing hybrid organic–inorganic halide perovskites (e.g., CH3NH3PbI3) have taken the photovoltaic community by storm. In the short time since being deemed its own class of emerging photovoltaic technologies by the National Renewable Energy Laboratory (October, 2013), the certified record efficiency of perovskite solar cells has increased nearly 50%, from 14.1 to 20.1% (http://www.nrel.gov/ncpv/). In addition, several groups have reported reproducible efficiencies in excess of 16%. These devices show great promise for commercial applications as they combine low-cost fabrication techniques with earth-abundant materials yet still deliver efficiencies rivaling traditional photovoltaic technologies. The possibility of using them in building facades or as a top cell in a tandem perovskite–Si architecture only increases their desirability. However, there is currently a dire need in the field for increased care on the part of authors in reporting their photovoltaic performance and on the side of reviewers and the scientific community at large in discriminating and evaluating reported results. Our hope is that this Viewpoint brings to light some of the issues pertaining to perovskite solar cells and provides the field with best practices for measuring and reporting perovskite solar cell performance.
Perovskite solar cells employing hybrid organic–inorganic halide perovskites (e.g., CH3NH3PbI3) have taken the photovoltaic community by storm. In the short time since being deemed its own class of emerging photovoltaic technologies by the National Renewable Energy Laboratory (October, 2013), the certified record efficiency of perovskite solar cells has increased nearly 50%, from 14.1 to 20.1% (http://www.nrel.gov/ncpv/). In addition, several groups have reported reproducible efficiencies in excess of 16%. These devices show great promise for commercial applications as they combine low-cost fabrication techniques with earth-abundant materials yet still deliver efficiencies rivaling traditional photovoltaic technologies. The possibility of using them in building facades or as a top cell in a tandem perovskite–Si architecture only increases their desirability. However, there is currently a dire need in the field for increased care on the part of authors in reporting their photovoltaic performance and on the side of reviewers and the scientific community at large in discriminating and evaluating reported results. Our hope is that this Viewpoint brings to light some of the issues pertaining to perovskite solar cells and provides the field with best practices for measuring and reporting perovskite solar cell performance.
475. Transformation of the Excited State and Photovoltaic Efficiency of CH3NH3PbI3 Perovskite upon Controlled Exposure to Humidified Air Christians, J. A.; Miranda Herrara, P. A.; Kamat, P. V. J. Am. Chem. Soc. 2015, 137(4),PP 1530-1538.
 Humidity has been an important factor, in both negative and positive ways, in the development of perovskite solar cells, and will prove critical in the push to commercialize this exciting new photovoltaic technology. The interaction between CH3NH3PbI3 and H2O vapor is investigated by characterizing the ground state and excited state optical absorption properties, and probing morphology and crystal structure. These systematic undertakings elucidate the complex interaction inherent in this system, demonstrating that H2O exposure does not simply only CH3NH3PbI3 to revert to PbI2. It is shown that, in the dark, H2O is able to complex with the perovskite, forming a hydrate product similar to (CH3NH3)4PbI6•2H2O. This causes a decrease in absorption across the visible region of the spectrum and a distinct change in the crystal structure of the material. Femtosecond transient absorption spectroscopic measurements show the effect that humidity has on the ultrafast excited state dynamics of CH3NH3PbI3. More importantly, the deleterious effects of humidity on complete solar cells, specifically on photovoltaic efficiency and stability, are explored in light of these spectroscopic understandings.
Humidity has been an important factor, in both negative and positive ways, in the development of perovskite solar cells, and will prove critical in the push to commercialize this exciting new photovoltaic technology. The interaction between CH3NH3PbI3 and H2O vapor is investigated by characterizing the ground state and excited state optical absorption properties, and probing morphology and crystal structure. These systematic undertakings elucidate the complex interaction inherent in this system, demonstrating that H2O exposure does not simply only CH3NH3PbI3 to revert to PbI2. It is shown that, in the dark, H2O is able to complex with the perovskite, forming a hydrate product similar to (CH3NH3)4PbI6•2H2O. This causes a decrease in absorption across the visible region of the spectrum and a distinct change in the crystal structure of the material. Femtosecond transient absorption spectroscopic measurements show the effect that humidity has on the ultrafast excited state dynamics of CH3NH3PbI3. More importantly, the deleterious effects of humidity on complete solar cells, specifically on photovoltaic efficiency and stability, are explored in light of these spectroscopic understandings.
474. All Solution-Processed Lead Halide Perovskite-BiVO4 Tandem Assembly for Photolytic Solar Fuels Production Chen, Y.-S.; Manser, J. S.; Kamat, P. V. J. Am. Chem. Soc. 2015, 137 (2), 974–981.
 The quest for economic, large scale hydrogen production has motivated the search for new materials and device designs capable of splitting water using only energy from the sun. Here we introduce an all solution-processed tandem water splitting assembly composed of a BiVO4 photoanode and a single-junction CH3NH3PbI3 hybrid perovskite solar cell. This unique configuration allows efficient solar photon management, with the metal oxide photoanode selectively harvesting high energy visible photons and the underlying perovskite solar cell capturing lower energy visible-near IR wavelengths in a single-pass excitation. Operating without external bias under standard AM 1.5G illumination, the photoanode-photovoltaic architecture, in conjunction with an earth-abundant cobalt phosphate catalyst, exhibits a solar-to-hydrogen conversion efficiency of 2.5% at neutral pH. The design of low-cost tandem water splitting assemblies employing single-junction hybrid perovskite materials establishes a potentially promising new frontier for solar water splitting research.
The quest for economic, large scale hydrogen production has motivated the search for new materials and device designs capable of splitting water using only energy from the sun. Here we introduce an all solution-processed tandem water splitting assembly composed of a BiVO4 photoanode and a single-junction CH3NH3PbI3 hybrid perovskite solar cell. This unique configuration allows efficient solar photon management, with the metal oxide photoanode selectively harvesting high energy visible photons and the underlying perovskite solar cell capturing lower energy visible-near IR wavelengths in a single-pass excitation. Operating without external bias under standard AM 1.5G illumination, the photoanode-photovoltaic architecture, in conjunction with an earth-abundant cobalt phosphate catalyst, exhibits a solar-to-hydrogen conversion efficiency of 2.5% at neutral pH. The design of low-cost tandem water splitting assemblies employing single-junction hybrid perovskite materials establishes a potentially promising new frontier for solar water splitting research.
473. Predicting the Rate Constant of Electron Tunneling Reactions at the CdSe-Linker-TiO2 Interface Hines, D. A.; Forrest, R. P.; Corcelli, S. A.; Kamat, P. V. J. Phys. Chem. B 2015, 119 (24), 7439-7446.
 Current interest in quantum dot solar cells (QDSCs) motivates an understanding of the electron transfer dynamics at the quantum dot (QD) – metal oxide (MO) interface. Employing transient absorption spectroscopy, we have monitored the electron transfer rate (ket) at this interface as a function of the bridge molecules that link QDs to TiO2. Using mercaptoacetic acid (MAA), 3-mercaptopropionic acid (3-MPA), 8-mercaptooctanoic acid (8-MOA) and 16-mercaptohexadecanoic acid (16-MHA) we observe an exponential attenuation of ket with increasing linker length, which has been attributed to the tunneling of the electron through the insulating linker molecule. We model the electron transfer reaction using both rectangular and trapezoidal barrier models that have been discussed in the literature. The one electron reduction potential (equivalent to the lowest unoccupied molecular orbital or LUMO) of each molecule as determined by cyclic voltammetry (CV) was used to estimate the effective barrier height presented by MAA, 3-MPA, 8-MOA and16-MHA at the CdSe-TiO2 interface. The electron transfer rate (ket) calculated for each CdSe-TiO2 interface using both models showed the results in agreement with the experimentally determined trend. This demonstrates that electron transfer between CdSe and TiO2 can be viewed as electron tunneling through a layer of organic linking molecules and provides a useful method for predicting electron transfer rate constants.
Current interest in quantum dot solar cells (QDSCs) motivates an understanding of the electron transfer dynamics at the quantum dot (QD) – metal oxide (MO) interface. Employing transient absorption spectroscopy, we have monitored the electron transfer rate (ket) at this interface as a function of the bridge molecules that link QDs to TiO2. Using mercaptoacetic acid (MAA), 3-mercaptopropionic acid (3-MPA), 8-mercaptooctanoic acid (8-MOA) and 16-mercaptohexadecanoic acid (16-MHA) we observe an exponential attenuation of ket with increasing linker length, which has been attributed to the tunneling of the electron through the insulating linker molecule. We model the electron transfer reaction using both rectangular and trapezoidal barrier models that have been discussed in the literature. The one electron reduction potential (equivalent to the lowest unoccupied molecular orbital or LUMO) of each molecule as determined by cyclic voltammetry (CV) was used to estimate the effective barrier height presented by MAA, 3-MPA, 8-MOA and16-MHA at the CdSe-TiO2 interface. The electron transfer rate (ket) calculated for each CdSe-TiO2 interface using both models showed the results in agreement with the experimentally determined trend. This demonstrates that electron transfer between CdSe and TiO2 can be viewed as electron tunneling through a layer of organic linking molecules and provides a useful method for predicting electron transfer rate constants.
472. Boosting the Photovoltage of Dye-Sensitized Solar Cells with Thiolated Gold Nanoclusters Choi, H.; Chen, Y.-S.; Stamplecoskie, K. G.; Kamat, P. V. J. Phys. Chem. Lett. 2015, 6 217-223.
 Glutathione-capped gold nanoclusters (Aux-GSH NCs) are anchored along with a sensitizing squaraine dye on a TiO2 surface to evaluate the cosensitizing role of Aux-GSH NCs in dye-sensitized solar cells (DSSCs). Photoelectrochemical measurements show an increase in the photoconversion efficiency of DSSCs when both sensitizers are present. The observed photoelectrochemical improvements in cosensitized DSSCs are more than additive effects as evident from the increase in photovoltage (ΔV as high as 0.24 V) when Aux-GSH NCs are present. Electron equilibration and accumulation within gold nanoclusters increase the quasi-Fermi level of TiO2 closer to the conduction band and thus decrease the photovoltage penalty. A similar beneficial role of gold nanoclusters toward boosting the Voc and enhancing the efficiency of Ru(II) polypyridyl complex-sensitized solar cells is also discussed.
Glutathione-capped gold nanoclusters (Aux-GSH NCs) are anchored along with a sensitizing squaraine dye on a TiO2 surface to evaluate the cosensitizing role of Aux-GSH NCs in dye-sensitized solar cells (DSSCs). Photoelectrochemical measurements show an increase in the photoconversion efficiency of DSSCs when both sensitizers are present. The observed photoelectrochemical improvements in cosensitized DSSCs are more than additive effects as evident from the increase in photovoltage (ΔV as high as 0.24 V) when Aux-GSH NCs are present. Electron equilibration and accumulation within gold nanoclusters increase the quasi-Fermi level of TiO2 closer to the conduction band and thus decrease the photovoltage penalty. A similar beneficial role of gold nanoclusters toward boosting the Voc and enhancing the efficiency of Ru(II) polypyridyl complex-sensitized solar cells is also discussed.
471. Surface Oxidation as a Cause of High Open-Circuit Voltage in CdSe ETA Solar Cells Kirmayer, S; Edri, E.; Hines, D.; Klein-Kedem, N.; Cohen, H.; Niitsoo, O.; Pinkas, I.; Kamat, P. V.; Hodes, G. Adv. Mater. Interf. 2015, ASAP.
 TiO2/CdSe/CuSCN extremely thin absorber (ETA) solar cells are found to give relatively high values of open-circuit voltage (>0.8 V) but low currents upon annealing the cadmium selenide (CdSe) in air (500 ºC). Annealing in N2 produces much lower photovoltages and slightly lower photocurrents. Band structure measurements show differences between the two annealing regimes that, however, appear to favor the N2-annealed CdSe. On the other hand, chemically resolved electrical measurements (CREM) of the cells reveal marked differences in photo-induced charge trapping, in particular at absorber grain boundaries of the air versus N2-annealed systems, correlated with the formation of Cd–O species at the CdSe surface. Using transient absorption and photovoltage decay, pronounced lifetime differences are also observed, in agreement with the strong suppression of charge recombination. The results point to a multiple role of grain surface-oxidation, which both impedes electron injection from the CdSe to the TiO2, but, much more significantly, enhances hole injection to the CuSCN via passivation of hole traps that act as efficient recombination centers.
TiO2/CdSe/CuSCN extremely thin absorber (ETA) solar cells are found to give relatively high values of open-circuit voltage (>0.8 V) but low currents upon annealing the cadmium selenide (CdSe) in air (500 ºC). Annealing in N2 produces much lower photovoltages and slightly lower photocurrents. Band structure measurements show differences between the two annealing regimes that, however, appear to favor the N2-annealed CdSe. On the other hand, chemically resolved electrical measurements (CREM) of the cells reveal marked differences in photo-induced charge trapping, in particular at absorber grain boundaries of the air versus N2-annealed systems, correlated with the formation of Cd–O species at the CdSe surface. Using transient absorption and photovoltage decay, pronounced lifetime differences are also observed, in agreement with the strong suppression of charge recombination. The results point to a multiple role of grain surface-oxidation, which both impedes electron injection from the CdSe to the TiO2, but, much more significantly, enhances hole injection to the CuSCN via passivation of hole traps that act as efficient recombination centers.
470. Dual Nature of the Excited State in Organic-Inorganic Lead Halide Perovskites Stamplecoskie, K. G.; Manser, J. S.; Kamat, P. V. Energy Environ. Sci. 2015, 8 (1), 208 - 215.
 The rapid increase in efficiency of methylammonium lead halide perovskite solar cells necessitates further investigation into the nature of perovskite absorption features and optical properties. Films obtained from the deposition of solutions containing lead halides and the CH3NH3+ organic cation is known to yield the CH3NH3PbI3 perovskite structure upon annealing. In examining the precursor solution used in the processing of CH3NH3PbI3 solar cells, we find that Pb2+ readily forms plumbate complexes in the presence of excess iodide ions and exhibits characteristic absorption bands at 370 (PbI3-) and 425 nm (PbI42-). Through comparative spectral analysis of the absorption features of charge transfer complexes in the solution phase and the final solid-state perovskite films, we are able to fully classify the absorption features in the excited state of CH3NH3PbI3 across the transient absorption spectrum recorded following laser pulse excitation. In particular, we attribute the broad photoinduced absorption to a charge-transfer excited state, and show correlation between the photoinduced absorption and 480 nm bleach signals. These observations lead us to propose a band structure composed of two distinct transitions that is consistent with the various spectral features and kinetic behavior of the CH3NH3PbI3 excited state. Characterization of this unique dual excited state nature provides further insight into the optoelectronic behavior of hybrid lead halide perovskite films and thus aids in elucidating their exceptional photovoltaic properties.
The rapid increase in efficiency of methylammonium lead halide perovskite solar cells necessitates further investigation into the nature of perovskite absorption features and optical properties. Films obtained from the deposition of solutions containing lead halides and the CH3NH3+ organic cation is known to yield the CH3NH3PbI3 perovskite structure upon annealing. In examining the precursor solution used in the processing of CH3NH3PbI3 solar cells, we find that Pb2+ readily forms plumbate complexes in the presence of excess iodide ions and exhibits characteristic absorption bands at 370 (PbI3-) and 425 nm (PbI42-). Through comparative spectral analysis of the absorption features of charge transfer complexes in the solution phase and the final solid-state perovskite films, we are able to fully classify the absorption features in the excited state of CH3NH3PbI3 across the transient absorption spectrum recorded following laser pulse excitation. In particular, we attribute the broad photoinduced absorption to a charge-transfer excited state, and show correlation between the photoinduced absorption and 480 nm bleach signals. These observations lead us to propose a band structure composed of two distinct transitions that is consistent with the various spectral features and kinetic behavior of the CH3NH3PbI3 excited state. Characterization of this unique dual excited state nature provides further insight into the optoelectronic behavior of hybrid lead halide perovskite films and thus aids in elucidating their exceptional photovoltaic properties.
2014

469. Size-Dependent Photovoltaic Performance of CuInS2 Quantum Dots Sensitized Solar Cells Jara, D. H.;Yoon, S.; Stamplecoskie, K. G.; Kamat, P. V. Chem. Mater. 2014, 26 (24), 7221–7228.
 The optical and electronic properties of quantum dots (QDs) which are drastically affected by their size have major impact on their performance in devices like solar cells. We now report the size dependent solar cell performance for CuInS2 QDs capped with 1-dodecanethiol. Pyramidal shaped CuInS2 QDs with diameter between 2.9 nm and 5.3 nm have been synthesized and assembled on mesoscopic TiO2 films by electrophoretic deposition. Time resolved emission and transient absorption spectroscopy measurements have ascertained the role of internal and surface defects in determining the solar cell performance. An increase in power conversion efficiency (PCE) was observed with increasing size of QDs, with maximum values of 2.14 and 2.51% for 3.9 and 4.3 nm size particles, respectively. The drop in PCE observed for larger QDs (5.3 nm) is attributed to decreased charge separation following bandgap excitation. Since the origin of photocurrent generation in CuInS2 QDSC arises from the defect dominated charge carriers it offers the opportunity to further improve the efficiency by controlling these defect concentrations.
The optical and electronic properties of quantum dots (QDs) which are drastically affected by their size have major impact on their performance in devices like solar cells. We now report the size dependent solar cell performance for CuInS2 QDs capped with 1-dodecanethiol. Pyramidal shaped CuInS2 QDs with diameter between 2.9 nm and 5.3 nm have been synthesized and assembled on mesoscopic TiO2 films by electrophoretic deposition. Time resolved emission and transient absorption spectroscopy measurements have ascertained the role of internal and surface defects in determining the solar cell performance. An increase in power conversion efficiency (PCE) was observed with increasing size of QDs, with maximum values of 2.14 and 2.51% for 3.9 and 4.3 nm size particles, respectively. The drop in PCE observed for larger QDs (5.3 nm) is attributed to decreased charge separation following bandgap excitation. Since the origin of photocurrent generation in CuInS2 QDSC arises from the defect dominated charge carriers it offers the opportunity to further improve the efficiency by controlling these defect concentrations.
468. The Origin of Catalytic Effect in the Reduction of CO2 at Nanostructured TiO2 Films Ramesha, G. K.; Brennecke, J. F.; Kamat, P. V. ACS Catal. 2014, 4 (9), 3249–3254.
 Electrocatalytic activity of mesoscopic TiO2 films towards the reduction of CO2 is probed by depositing a nanostructured film on a glassy carbon electrode. The one-electron reduction of CO2 in acetonitrile seen at an onset potential of -1.1 V (vs. NHE) is ~0.5 V lower than the one observed with a glassy carbon electrode. The electrocatalytic role of TiO2 is elucidated through spectroelectrochemistry and product analysis. Ti3+ species formed when TiO2 film is subjected to negative potentials have been identified as active reduction sites. Binding of CO2 to catalytically active Ti3+ followed by the electron transfer facilitates the initial one-electron reduction process. Methanol was the primary product when the reduction was carried out in wet acetonitrile.
Electrocatalytic activity of mesoscopic TiO2 films towards the reduction of CO2 is probed by depositing a nanostructured film on a glassy carbon electrode. The one-electron reduction of CO2 in acetonitrile seen at an onset potential of -1.1 V (vs. NHE) is ~0.5 V lower than the one observed with a glassy carbon electrode. The electrocatalytic role of TiO2 is elucidated through spectroelectrochemistry and product analysis. Ti3+ species formed when TiO2 film is subjected to negative potentials have been identified as active reduction sites. Binding of CO2 to catalytically active Ti3+ followed by the electron transfer facilitates the initial one-electron reduction process. Methanol was the primary product when the reduction was carried out in wet acetonitrile.
467. Band Filling with Free Charge Carriers in Organometal Halide Perovskites Manser, J. S.; Kamat, P. V. Nat. Photon. 2014, 8, 737–743.
 The unique and promising properties of semiconducting organometal halide perovskites have brought these materials to the forefront of solar energy research. Here, we present new insights into the excited-state properties of CH3>NH3PbI3 thin films through femtosecond transient absorption spectroscopy measurements. The photoinduced bleach recovery at 760 nm reveals that band-edge recombination follows second-order kinetics, indicating that the dominant relaxation pathway is via recombination of free electrons and holes. Additionally, charge accumulation in the perovskite films leads to an increase in the intrinsic bandgap that follows the Burstein–Moss band filling model. Both the recombination mechanism and the band-edge shift are studied as a function of the photogenerated carrier density and serve to elucidate the behaviour of charge carriers in hybrid perovskites. These results offer insights into the intrinsic photophysics of semiconducting organometal halide perovskites with direct implications for photovoltaic and optoelectronic applications.
The unique and promising properties of semiconducting organometal halide perovskites have brought these materials to the forefront of solar energy research. Here, we present new insights into the excited-state properties of CH3>NH3PbI3 thin films through femtosecond transient absorption spectroscopy measurements. The photoinduced bleach recovery at 760 nm reveals that band-edge recombination follows second-order kinetics, indicating that the dominant relaxation pathway is via recombination of free electrons and holes. Additionally, charge accumulation in the perovskite films leads to an increase in the intrinsic bandgap that follows the Burstein–Moss band filling model. Both the recombination mechanism and the band-edge shift are studied as a function of the photogenerated carrier density and serve to elucidate the behaviour of charge carriers in hybrid perovskites. These results offer insights into the intrinsic photophysics of semiconducting organometal halide perovskites with direct implications for photovoltaic and optoelectronic applications.
466. Is Graphene a Stable Platform for Photocatalysis? Mineralization of Reduced Graphene Oxide with UV-Irradiated TiO2 Nanoparticles Radich J. G.; Krenselewski, A.; Zhu, J.; Kamat, P. V. Chem. Mater. 2014, 26 (15), 4662–4668.
 The recent thrust in utilizing reduced graphene oxide (RGO) as a support for nanostructured catalyst particles has led to the claims of improved efficiency in solar cells, fuel cells, and photocatalytic degradation of pollutants. Specifically, the robust TiO2 system is often coupled with RGO to improve charge separation and facilitate redox reactions. Here we probe the stability of RGO in the presence of UV-excited TiO2 in aqueous media and establish its reactivity towards OH• radicals, a primary oxidant generated at the TiO2 surface. By probing changes in absorption, morphology and total organic carbon content (TOC) we conclusively demonstrate the vulnerability of RGO towards OH• attack and raise the concern of its use in many applications where OH• are likely to be formed. On the other hand, the OH• radical-mediated mineralization could also enable new approaches in tackling environmental remediation of nanocarbons such as RGO, carbon nanotubes, and fullerenes.
The recent thrust in utilizing reduced graphene oxide (RGO) as a support for nanostructured catalyst particles has led to the claims of improved efficiency in solar cells, fuel cells, and photocatalytic degradation of pollutants. Specifically, the robust TiO2 system is often coupled with RGO to improve charge separation and facilitate redox reactions. Here we probe the stability of RGO in the presence of UV-excited TiO2 in aqueous media and establish its reactivity towards OH• radicals, a primary oxidant generated at the TiO2 surface. By probing changes in absorption, morphology and total organic carbon content (TOC) we conclusively demonstrate the vulnerability of RGO towards OH• attack and raise the concern of its use in many applications where OH• are likely to be formed. On the other hand, the OH• radical-mediated mineralization could also enable new approaches in tackling environmental remediation of nanocarbons such as RGO, carbon nanotubes, and fullerenes.
465. Size-Dependent Excited State Behavior of Glutathione-Capped Gold Clusters and Their Light-Harvesting Capacity Stamplecoskie, K. G.; Kamat, P. V. J. Am. Chem. Soc. 2014, 136 (31), 11093–11099.
 Glutathione protected gold clusters exhibit size dependent excited state and electron transfer properties. Larger size clusters (e.g., Au25GSH18) with core-metal atoms display rapid (<1 ps) as well as slower relaxation (~200 ns) while homoleptic clusters (e.g., Au10-12GSH10-12) exhibit only slower relaxation. These decay components have been identified as metal-metal transition and ligand-to-metal charge transfer respectively. The short lifetime relaxation component becomes less dominant as the size of the gold cluster decreases. The long-lived excited state and ability to participate in electron transfer are integral for these clusters to serve as light harvesting antennae. A strong correlation between the ligand-to-metal charge-transfer excited state lifetime and photocatalytic activity was evidenced from the electron transfer to methyl viologen. The photoactivity of these metal clusters show increasing photocatalytic reduction yield (0.05 - 0.14) with decreasing cluster size, Au25 < Au18 < Au15 < Au10-12. Gold clusters, Au18GSH14, were found to have the highest potential as a photosensitizer based on the quantum yield of electron transfer and good visible light absorption properties.
Glutathione protected gold clusters exhibit size dependent excited state and electron transfer properties. Larger size clusters (e.g., Au25GSH18) with core-metal atoms display rapid (<1 ps) as well as slower relaxation (~200 ns) while homoleptic clusters (e.g., Au10-12GSH10-12) exhibit only slower relaxation. These decay components have been identified as metal-metal transition and ligand-to-metal charge transfer respectively. The short lifetime relaxation component becomes less dominant as the size of the gold cluster decreases. The long-lived excited state and ability to participate in electron transfer are integral for these clusters to serve as light harvesting antennae. A strong correlation between the ligand-to-metal charge-transfer excited state lifetime and photocatalytic activity was evidenced from the electron transfer to methyl viologen. The photoactivity of these metal clusters show increasing photocatalytic reduction yield (0.05 - 0.14) with decreasing cluster size, Au25 < Au18 < Au15 < Au10-12. Gold clusters, Au18GSH14, were found to have the highest potential as a photosensitizer based on the quantum yield of electron transfer and good visible light absorption properties.
464. Size-Dependent Energy Transfer Pathways in CdSe Quantum Dot–Squaraine Light-Harvesting Assemblies: Förster versus Dexter Hoffman, J. B.; Choi, H.; Kamat, P. V. J. Phys. Chem. C 2014, 118 (32), 18453–18461.
 Energy transfer coupled with electron transfer is a convenient approach to mimic photosynthesis in light energy conversion. Better understanding of mechanistic details of energy transfer processes is important to enhance the performance of dye or quantum dot sensitized solar cells. Energy transfer through both long range dipole based Förster Resonance Energy Transfer (FRET), and short range Dexter Energy Transfer (DET) mechanisms have been identified to occur between CdSe quantum dots (QDs) linked to a red-infrared absorbing squaraine dye through a short thiol functional group (SQSH). Solutions of SQSH linked to CdSe were investigated through steady-state and time resolved spectroscopy experiments to explore both mechanisms. Photoluminescence studies revealed that smaller QDs had higher energy transfer efficiencies than predicted by FRET, and femtosecond transient absorption experiments revealed faster energy transfer rates in smaller donor QD sizes. These findings supported A DET process dominating at small donor sizes. The presence of both processes illustrates multiple strategies for utilizing energy transfer in light harvesting assemblies and the required considerations in device design to maximize energy transfer gains through either mechanism.
Energy transfer coupled with electron transfer is a convenient approach to mimic photosynthesis in light energy conversion. Better understanding of mechanistic details of energy transfer processes is important to enhance the performance of dye or quantum dot sensitized solar cells. Energy transfer through both long range dipole based Förster Resonance Energy Transfer (FRET), and short range Dexter Energy Transfer (DET) mechanisms have been identified to occur between CdSe quantum dots (QDs) linked to a red-infrared absorbing squaraine dye through a short thiol functional group (SQSH). Solutions of SQSH linked to CdSe were investigated through steady-state and time resolved spectroscopy experiments to explore both mechanisms. Photoluminescence studies revealed that smaller QDs had higher energy transfer efficiencies than predicted by FRET, and femtosecond transient absorption experiments revealed faster energy transfer rates in smaller donor QD sizes. These findings supported A DET process dominating at small donor sizes. The presence of both processes illustrates multiple strategies for utilizing energy transfer in light harvesting assemblies and the required considerations in device design to maximize energy transfer gains through either mechanism.
463. Sense and Shoot: Simultaneous Detection and Degradation of Low Level Contaminants using Graphene Based Smart Material Assembly Alam, R.; Lightcap, I. V.; Karwacki, C. J.; Kamat, P. V. ACS Nano 2014, 8 (7), 7272–7278.
 Smart material nanoassemblies that can simultaneously sense and shoot low level contaminants from air and water are important for overcoming the threat of hazardous chemicals. Graphene oxide (GO) sheets deposited on mesoscopic TiO2 films that underpin the deposition of Ag nanoparticles with UV-irradiation provide the foundation for the design of a smart material. The Ag particle size is readily controlled through precursor concentration and UV irradiation time. These semiconductor – graphene oxide – metal (SGM) films are SERS active and hence capable of sensing aromatic contaminants such as nitrobenzenethiol (NBT) in nanomolar range. Increased local concentration of organic molecule achieved through interaction with 2D carbon support (GO) facilitates low-level detection of contaminants. Upon UV irradiation of the NBT loaded SGM film, one can induce photocatalytic transformations. Thus, each component of the SGM film plays a pivotal role in aiding the detection and degradation of a contaminant dispersed in aqueous solutions. The advantage of using SGM films as multipurpose "detect and destroy" systems for nitroaromatic molecule is discussed.
Smart material nanoassemblies that can simultaneously sense and shoot low level contaminants from air and water are important for overcoming the threat of hazardous chemicals. Graphene oxide (GO) sheets deposited on mesoscopic TiO2 films that underpin the deposition of Ag nanoparticles with UV-irradiation provide the foundation for the design of a smart material. The Ag particle size is readily controlled through precursor concentration and UV irradiation time. These semiconductor – graphene oxide – metal (SGM) films are SERS active and hence capable of sensing aromatic contaminants such as nitrobenzenethiol (NBT) in nanomolar range. Increased local concentration of organic molecule achieved through interaction with 2D carbon support (GO) facilitates low-level detection of contaminants. Upon UV irradiation of the NBT loaded SGM film, one can induce photocatalytic transformations. Thus, each component of the SGM film plays a pivotal role in aiding the detection and degradation of a contaminant dispersed in aqueous solutions. The advantage of using SGM films as multipurpose "detect and destroy" systems for nitroaromatic molecule is discussed.
462. Switching the Reaction Course of Electrochemical CO2 Reduction with Ionic Liquids Sun, L.; Ramesha, G. K.; Kamat, P. V.; Brennecke, J. F. Langmuir 2014, 30 (21), 6302–6308.
 The ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([emim][Tf2N]) offers new ways to modulate the electrochemical reduction of carbon dioxide. [emim][Tf2N], when present as the supporting electrolyte in acetonitrile, decreases the reduction overpotential at a Pb electrode by 0.18 V as compared to tetraethylammonium perchlorate as the supporting electrolyte. More interestingly, the ionic liquid shifts the reaction course during the electrochemical reduction of carbon dioxide by promoting the formation of carbon monoxide instead of oxalate anion. With increasing concentration of [emim][Tf2N], a carboxylate species with reduced CO2 covalently bonded to the imidazolium ring is formed along with carbon monoxide. The results highlight the catalytic effects of the medium in modulating the CO2 reduction products.
The ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([emim][Tf2N]) offers new ways to modulate the electrochemical reduction of carbon dioxide. [emim][Tf2N], when present as the supporting electrolyte in acetonitrile, decreases the reduction overpotential at a Pb electrode by 0.18 V as compared to tetraethylammonium perchlorate as the supporting electrolyte. More interestingly, the ionic liquid shifts the reaction course during the electrochemical reduction of carbon dioxide by promoting the formation of carbon monoxide instead of oxalate anion. With increasing concentration of [emim][Tf2N], a carboxylate species with reduced CO2 covalently bonded to the imidazolium ring is formed along with carbon monoxide. The results highlight the catalytic effects of the medium in modulating the CO2 reduction products.
461. How Does a SILAR CdSe Film Grow? Tuning the Deposition Steps to Suppress Interfacial Charge Recombination in Solar Cells Becker, M. A.; Radich, J. G.; Bunker, B. A.; Kamat, P. V. J. Phys Chem. Lett. 2014, 5, 1575–1582.
 Successive Ionic Layer Adsorption and Reaction (SILAR) is a popular method of depositing the metal chalcogenide semiconductor layer on the mesoscopic metal oxide films for designing quantum dot sensitized solar cell (QDSSC) or Extremely Thin Absorber (ETA) solar cells. While this deposition method exhibits higher loading of light absorbing semiconductor layer than direct adsorption of pre-synthesized colloidal quantum dots, the chemical identity of these nanostructures and evolution of interfacial structure are poorly understood. We have now analyzed step-by-step SILAR deposition of CdSe films on mesoscopic TiO2 nanoparticle films using x-ray absorption near-edge structure analysis and probed the interfacial structure of these films. The film characteristics interestingly show dependence on the order in which the Cd and Se are deposited, and the CdSe-TiO2 interface is affected only during the first few cycles of deposition. Development of SeO2 passivation layer in the SILAR-prepared films to form TiO2/SeO2/CdSe junction facilitates an increase in photocurrents and power conversion efficiencies of quantum dot solar cells when these films were integrated as photoanodes in a photoelectrochemical solar cell.
Successive Ionic Layer Adsorption and Reaction (SILAR) is a popular method of depositing the metal chalcogenide semiconductor layer on the mesoscopic metal oxide films for designing quantum dot sensitized solar cell (QDSSC) or Extremely Thin Absorber (ETA) solar cells. While this deposition method exhibits higher loading of light absorbing semiconductor layer than direct adsorption of pre-synthesized colloidal quantum dots, the chemical identity of these nanostructures and evolution of interfacial structure are poorly understood. We have now analyzed step-by-step SILAR deposition of CdSe films on mesoscopic TiO2 nanoparticle films using x-ray absorption near-edge structure analysis and probed the interfacial structure of these films. The film characteristics interestingly show dependence on the order in which the Cd and Se are deposited, and the CdSe-TiO2 interface is affected only during the first few cycles of deposition. Development of SeO2 passivation layer in the SILAR-prepared films to form TiO2/SeO2/CdSe junction facilitates an increase in photocurrents and power conversion efficiencies of quantum dot solar cells when these films were integrated as photoanodes in a photoelectrochemical solar cell.
460. Glutathione Capped Gold Nanoclusters as Photosensitizers. Visible Light Induced Hydrogen Generation in Neutral Water Chen, Y.-S.; Kamat, P. V. J. Am. Chem. Soc. 2014, 136 (16), 6075–6082.
 Glutathione capped metal nanoclusters (Aux-GSH NCs) which exhibit molecular like properties are employed as a photosen-sitizer for hydrogen generation in a photoelectrochemical cell (PEC) and a photocatalytic slurry reactor. The reversible re-duction (E0= -0.63 V vs. RHE) and oxidation (E0= 0.97 V and 1.51 V vs. RHE) potentials of these metal nanoclusters make them suita-ble for driving the water splitting reaction. When a mesoscopic TiO2 film sensitized by Aux-GSH NCs was used as the pho-toanode with a Pt counter electrode in aqueous buffer solution (pH = 7), we observe significant photocurrent activity under visible light (400 - 500 nm) excitation. Additionally, sensitizing Pt/TiO2 nanoparticles with Aux-GSH NCs in an aqueous slurry system and irradiating with visible light produced H2 at a rate of 0.3 mmole of hydrogen/hr/gram of Aux-GSH NCs. The rate of H2 evolution was significantly enhanced (~5 times) when a sacrificial donor, such as EDTA, was introduced into the sys-tem. Using metal nanoclusters as a photosensitizer for hydrogen generation lays the foundation for the future exploration of other metal nanoclusters with well controlled numbers of metal atoms and capping ligands.
Glutathione capped metal nanoclusters (Aux-GSH NCs) which exhibit molecular like properties are employed as a photosen-sitizer for hydrogen generation in a photoelectrochemical cell (PEC) and a photocatalytic slurry reactor. The reversible re-duction (E0= -0.63 V vs. RHE) and oxidation (E0= 0.97 V and 1.51 V vs. RHE) potentials of these metal nanoclusters make them suita-ble for driving the water splitting reaction. When a mesoscopic TiO2 film sensitized by Aux-GSH NCs was used as the pho-toanode with a Pt counter electrode in aqueous buffer solution (pH = 7), we observe significant photocurrent activity under visible light (400 - 500 nm) excitation. Additionally, sensitizing Pt/TiO2 nanoparticles with Aux-GSH NCs in an aqueous slurry system and irradiating with visible light produced H2 at a rate of 0.3 mmole of hydrogen/hr/gram of Aux-GSH NCs. The rate of H2 evolution was significantly enhanced (~5 times) when a sacrificial donor, such as EDTA, was introduced into the sys-tem. Using metal nanoclusters as a photosensitizer for hydrogen generation lays the foundation for the future exploration of other metal nanoclusters with well controlled numbers of metal atoms and capping ligands.
459. CdSe-Graphene Oxide Light Harvesting Assembly. Size Dependent Electron Transfer and Light Energy Conversion Aspects Krishnamurthy, S.; Kamat, P. V. ChemPhysChem 2014, 15, 2129–2135.
 Excited state interaction between CdSe quantum dots (QD) of different sizes (2.3, 3.2 and 4.2 nm diameter) and graphene oxide (GO) has been probed by depositing them as films on conducting glass electrodes. The emission of smaller CdSe QDs (2.3 nm) is quenched by GO three times faster than that of larger QDs (4.2 nm). Electrophoretic deposition method has allowed us to sequentially deposit single or multiple layers of different sized QDs and GO assemblies on conducting glass electrode and achieve the modulation of photoresponse in photoelectrochemical solar cells. Superior photoconversion efficiency through the incorporation of GO is attributed to the improved charge separation in the composite assembly.
Excited state interaction between CdSe quantum dots (QD) of different sizes (2.3, 3.2 and 4.2 nm diameter) and graphene oxide (GO) has been probed by depositing them as films on conducting glass electrodes. The emission of smaller CdSe QDs (2.3 nm) is quenched by GO three times faster than that of larger QDs (4.2 nm). Electrophoretic deposition method has allowed us to sequentially deposit single or multiple layers of different sized QDs and GO assemblies on conducting glass electrode and achieve the modulation of photoresponse in photoelectrochemical solar cells. Superior photoconversion efficiency through the incorporation of GO is attributed to the improved charge separation in the composite assembly.

458. Quantum Dot Solar Cells. Hole Transfer as a Limiting Factor in Boosting Photoconversion Efficiency Kamat, P. V.; Christians, J. A.; Radich, J. G. Langmuir 30 (20), 5716-5725 (Feature Article).
 Semiconductor nanostructures are attractive for designing low cost solar cells with tunable photoresponse. The recent advances in size and shape selective synthesis have enabled the design of quantum dot solar cells with photoconversion efficiencies greater than 5%. In order to make them competitive with other existing thin film or polycrystalline photovoltaic technologies, it is important to overcome kinetic barriers for charge transfer at semiconductor interfaces. This feature focuses on the limitations imposed by slow hole transfer in improving solar cell performance and its role in the stability of metal chalcogenide solar cells. Strategies to improve the rate of hole transfer through surface modified redox relays offer new opportunities to overcome the hole transfer limitation. The mechanistic and kinetic aspects of hole transfer in Quantum Dot Solar Cells (QDSCs), Nanowire Solar Cells (NWSCs) and Extremely Thin Absorber (ETA) solar cells are discussed.
Semiconductor nanostructures are attractive for designing low cost solar cells with tunable photoresponse. The recent advances in size and shape selective synthesis have enabled the design of quantum dot solar cells with photoconversion efficiencies greater than 5%. In order to make them competitive with other existing thin film or polycrystalline photovoltaic technologies, it is important to overcome kinetic barriers for charge transfer at semiconductor interfaces. This feature focuses on the limitations imposed by slow hole transfer in improving solar cell performance and its role in the stability of metal chalcogenide solar cells. Strategies to improve the rate of hole transfer through surface modified redox relays offer new opportunities to overcome the hole transfer limitation. The mechanistic and kinetic aspects of hole transfer in Quantum Dot Solar Cells (QDSCs), Nanowire Solar Cells (NWSCs) and Extremely Thin Absorber (ETA) solar cells are discussed.
457. CdSeS Nanowires. Compositionally Controlled Band Gap and Exciton Dynamics Kim J.-P.; Christians, J. A.; Choi, H.; Krishnamurthy, S.; Kamat, P. V. J. Phys. Chem. Lett. 5, 1103-1109.
 CdS, CdSe and ternary CdSexS(1-x) are some of the most widely studied II-VI semiconductors due to their wide range of applications and promising performance in numerous systems. One-dimensional semiconductor nanowires offer the ability to conduct charges efficiently along the length of the wire which has potential charge transport benefits compared to nanoparticles. Herein, we report a simple, inexpensive synthetic procedure for high quality CdSeS nanowires where the composition can be easily modulated from pure CdSe to pure CdS by simply adjusting the Se:S precursor ratio. This allows for tuning of the absorption and emission properties of the nanowires across the visible spectrum. The CdSeS nanowires have a wurtzite crystal structure and grow along the [001] direction. As measured by femtosecond transient absorption spectroscopy, the short component of the excited state lifetime remains relatively constant at ~10 ps with increasing Se; however the contribution of this short lifetime component increased dramatically from 8.4% to 57.7% with increasing Se content. These CdSeS nanowires offer facile synthesis and widely adjustable optical properties, characteristics which give them broad potential applications in the fields of optoelectronics, and photovoltaics.
CdS, CdSe and ternary CdSexS(1-x) are some of the most widely studied II-VI semiconductors due to their wide range of applications and promising performance in numerous systems. One-dimensional semiconductor nanowires offer the ability to conduct charges efficiently along the length of the wire which has potential charge transport benefits compared to nanoparticles. Herein, we report a simple, inexpensive synthetic procedure for high quality CdSeS nanowires where the composition can be easily modulated from pure CdSe to pure CdS by simply adjusting the Se:S precursor ratio. This allows for tuning of the absorption and emission properties of the nanowires across the visible spectrum. The CdSeS nanowires have a wurtzite crystal structure and grow along the [001] direction. As measured by femtosecond transient absorption spectroscopy, the short component of the excited state lifetime remains relatively constant at ~10 ps with increasing Se; however the contribution of this short lifetime component increased dramatically from 8.4% to 57.7% with increasing Se content. These CdSeS nanowires offer facile synthesis and widely adjustable optical properties, characteristics which give them broad potential applications in the fields of optoelectronics, and photovoltaics.
456. Carbon Nanohoops: Excited Singlet and Triplet Behavior of [9]- and [12]-cycloparaphenylene Hines, D. A.; Darzi, E. R.; Jasti, R.; Kamat, P. V. J. Phys. Chem. A 2014, 118 (9), 1595–1600.
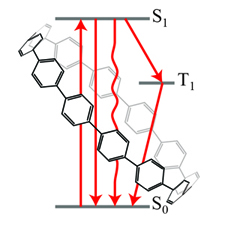 Cycloparaphenylene molecules, commonly known as 'carbon nanohoops,' have the potential to serve as building blocks in constructing carbon nanotube architectures. The singlet and triplet excited state characteristics of [9]-cycloparaphenylene ([9]CPP) and [12]-cycloparaphenylene ([12]CPP) have now been elucidated using time resolved transient absorption and emission techniques. The fluorescence quantum yields (Φ) of [9]CPP and [12]CPP were determined to be 0.46 and 0.83 respectively. Rates of non-radiative recombination (knr), radiative recombination (kr) and intersystem crossing (kisc) determined in this study indicate that radiative decay dominates in these nanohoop structures. The triplet quantum yields determined through energy transfer with excited biphenyl triplet were 0.18 and 0.13 for [9]CPP and [12]CPP respectively. The rate of triplet state quenching by oxygen was measured to be 1.7 × 103 s-1 ([9]CPP) and 1.9 × 103 s-1 ([12]CPP). The excited state dynamics established in this study enable us to understand the behavior of a carbon nanotube-like structure on a single subunit level.
Cycloparaphenylene molecules, commonly known as 'carbon nanohoops,' have the potential to serve as building blocks in constructing carbon nanotube architectures. The singlet and triplet excited state characteristics of [9]-cycloparaphenylene ([9]CPP) and [12]-cycloparaphenylene ([12]CPP) have now been elucidated using time resolved transient absorption and emission techniques. The fluorescence quantum yields (Φ) of [9]CPP and [12]CPP were determined to be 0.46 and 0.83 respectively. Rates of non-radiative recombination (knr), radiative recombination (kr) and intersystem crossing (kisc) determined in this study indicate that radiative decay dominates in these nanohoop structures. The triplet quantum yields determined through energy transfer with excited biphenyl triplet were 0.18 and 0.13 for [9]CPP and [12]CPP respectively. The rate of triplet state quenching by oxygen was measured to be 1.7 × 103 s-1 ([9]CPP) and 1.9 × 103 s-1 ([12]CPP). The excited state dynamics established in this study enable us to understand the behavior of a carbon nanotube-like structure on a single subunit level.
455. Charge Transfer Mediation Through CuxS. The Hole Story of CdSe in Polysulfide Radich, J.G.; Peeples, N. R.; Santra, P. K.; Kamat, P. V. J. Phys. Chem. C 2014, 118 (30), 16463–16471.
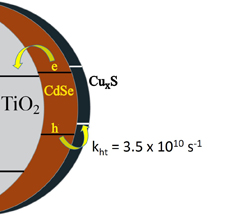 Hole transfer to dissolved sulfide species in liquid junction CdSe quantum dot sensitized solar cells is relatively slow when compared to electron transfer from CdSe to TiO2. Controlled exposure of cadmium chalcogenide surfaces to copper ions followed by immersion in sulfide solution promotes development of interfacial CuxS layer, which mediates hole transfer to polysulfide electrolyte by collection of photogenerated holes from CdSe. In addition, CuxS was also found to interact directly with defect states on the CdSe surface and quench emission characteristic of electron traps resulting from selenide vacancies. Together these effects were found to work in tandem to deliver 6.6% power conversion efficiency using Mn-doped CdS and CdSe co-sensitized quantum dot solar cell. Development of n-p interfacial junction at the photoanode-electrolyte interface in quantum dot solar cells unveils new means for designing high efficiency liquid junction solar cells.
Hole transfer to dissolved sulfide species in liquid junction CdSe quantum dot sensitized solar cells is relatively slow when compared to electron transfer from CdSe to TiO2. Controlled exposure of cadmium chalcogenide surfaces to copper ions followed by immersion in sulfide solution promotes development of interfacial CuxS layer, which mediates hole transfer to polysulfide electrolyte by collection of photogenerated holes from CdSe. In addition, CuxS was also found to interact directly with defect states on the CdSe surface and quench emission characteristic of electron traps resulting from selenide vacancies. Together these effects were found to work in tandem to deliver 6.6% power conversion efficiency using Mn-doped CdS and CdSe co-sensitized quantum dot solar cell. Development of n-p interfacial junction at the photoanode-electrolyte interface in quantum dot solar cells unveils new means for designing high efficiency liquid junction solar cells.

454. Recent Advances in Quantum Dot Surface Chemistry Hines, D. A.; Kamat, P. V. ACS Appl. Mater. Interfaces 2014, 6 (5), 3041–3057.
 Quantum dot (QD) surface chemistry is an emerging field in semiconductor nanocrystal related research. Along with size manipulation, the careful control of QD surface chemistry allows modulation of the optical properties of a QD suspension. Even a single molecule bound to the surface can introduce new functionalities. Herein, we summarize the recent advances in QD surface chemistry and the resulting effects on optical and electronic properties. Specifically, the feature article focuses addresses three main issues: (i) How surface chemistry affects the optical properties of QDs, (ii) How it influences the excited state dynamics and (iii) How one can manipulate surface chemistry to control the interactions between QDs and metal oxides, metal nanoparticles and in self-assembled QD monolayers.
Quantum dot (QD) surface chemistry is an emerging field in semiconductor nanocrystal related research. Along with size manipulation, the careful control of QD surface chemistry allows modulation of the optical properties of a QD suspension. Even a single molecule bound to the surface can introduce new functionalities. Herein, we summarize the recent advances in QD surface chemistry and the resulting effects on optical and electronic properties. Specifically, the feature article focuses addresses three main issues: (i) How surface chemistry affects the optical properties of QDs, (ii) How it influences the excited state dynamics and (iii) How one can manipulate surface chemistry to control the interactions between QDs and metal oxides, metal nanoparticles and in self-assembled QD monolayers.
453. Driving Charge Separation for Hybrid Solar Cells: Photo-induced Hole Transfer in Conjugated Copolymer and Semiconductor Nanoparticle Assemblies Wang, Y.; Liu, K.; Mikherjee, P.; Hines, D. A.; Santra, P.; Shen, H. Y.; Kamat, P. V.; Waldeck, D. H. Phys. Chem. Chem. Phys. 2014, 16, 5066-5070.
 This work reports on the use of an internal electrostatic field to facilitate charge separation at inorganic-organic interfaces, analogous to those in hybrid solar cells. Systematic charge transfer studies show that the donor-acceptor charge transfer rate is highly sensitive to the direction of the internal electric field.
This work reports on the use of an internal electrostatic field to facilitate charge separation at inorganic-organic interfaces, analogous to those in hybrid solar cells. Systematic charge transfer studies show that the donor-acceptor charge transfer rate is highly sensitive to the direction of the internal electric field.
452. Rate Limiting Interfacial Hole Transfer in Sb2S3 Solid-State Solar Cells Christians, J. A.; Leighton Jr., D. T.; Kamat, P. V. Energy and Environ. Sci. 2014, 7, 1148-1158.
 Transfer of photogenerated holes from the absorber species to the p-type hole conductor is fundamental to the performance of solid-state sensitized solar cells. In this study, we comprehensively investigate hole diffusion in the Sb2S3 absorber and hole transfer across the Sb2S3–CuSCN interface in the TiO2/Sb2S3/CuSCN system using femtosecond transient absorption spectroscopy, carrier diffusion modeling, and photovoltaic performance studies. Transfer of photogenerated holes from Sb2S3 to CuSCN is found to be dependent on Sb2S3 film thickness, a trend attributed to diffusion in the Sb2S3 absorber. However, modeling reveals that this process is not adequately described by diffusion limitations alone as has been assumed in similar systems. Therefore, both diffusion and transfer across the Sb2S3–CuSCN interface are taken into account to describe the hole transfer dynamics. Modeling of diffusion and interfacial hole transfer effects reveal that interfacial hole transfer, not diffusion, is the predominate factor dictating the magnitude of the hole transfer rate, especially in thin (< 20 nm) Sb2S3 films. Lastly, the implications of these results are further explored by photovoltaic measurements using planar TiO2/Sb2S3/CuSCN solar cells to elucidate the role of hole transfer in photovoltaic performance.
Transfer of photogenerated holes from the absorber species to the p-type hole conductor is fundamental to the performance of solid-state sensitized solar cells. In this study, we comprehensively investigate hole diffusion in the Sb2S3 absorber and hole transfer across the Sb2S3–CuSCN interface in the TiO2/Sb2S3/CuSCN system using femtosecond transient absorption spectroscopy, carrier diffusion modeling, and photovoltaic performance studies. Transfer of photogenerated holes from Sb2S3 to CuSCN is found to be dependent on Sb2S3 film thickness, a trend attributed to diffusion in the Sb2S3 absorber. However, modeling reveals that this process is not adequately described by diffusion limitations alone as has been assumed in similar systems. Therefore, both diffusion and transfer across the Sb2S3–CuSCN interface are taken into account to describe the hole transfer dynamics. Modeling of diffusion and interfacial hole transfer effects reveal that interfacial hole transfer, not diffusion, is the predominate factor dictating the magnitude of the hole transfer rate, especially in thin (< 20 nm) Sb2S3 films. Lastly, the implications of these results are further explored by photovoltaic measurements using planar TiO2/Sb2S3/CuSCN solar cells to elucidate the role of hole transfer in photovoltaic performance.
451. Kinetics and Mechanism of •OH Mediated Degradation of Dimethyl Phthalate in Aqueous Solution: Experimental and Theoretical Studies An T.; Gao, Y.; Li, G.; Kamat, P. V.; Peller, J.; Joyce, M. V. Environ. Sci. Technol. 2014, 48 (1), 641–648.
 The hydroxyl radical (•OH) is one of the main oxidative species in aqueous phase advanced oxidation processes, and its initial reactions with organic pollutants are important to understand the transformation and fate of organics in water environments. Insights into the kinetics and mechanism of •OH mediated degradation of the model environmental endocrine disruptor, dimethyl phthalate (DMP), have been obtained using radiolysis experiments and computational methods. The bimolecular rate constant for the •OH reaction with DMP was determined to be (3.2 ± 0.1) × 109 M–1s–1. The possible reaction mechanisms of radical adduct formation (RAF), hydrogen atom transfer (HAT), and single electron transfer (SET) were considered. By comparing the experimental absorption spectra with the computational results, it was concluded that the RAF and HAT were the dominant reaction pathways, and OH-adducts (•DMPOH1, •DMPOH2) and methyl type radicals •DMP(-H)α were identified as dominated intermediates. Computational results confirmed the identification of transient species with maximum absorption around 260 nm as •DMPOH1 and •DMP(-H)α, and these radical intermediates then converted to monohydroxylated dimethyl phthalates and monomethyl phthalates. Experimental and computational analyses which elucidated the mechanism of •OH-mediated degradation of DMP are discussed in detail.
The hydroxyl radical (•OH) is one of the main oxidative species in aqueous phase advanced oxidation processes, and its initial reactions with organic pollutants are important to understand the transformation and fate of organics in water environments. Insights into the kinetics and mechanism of •OH mediated degradation of the model environmental endocrine disruptor, dimethyl phthalate (DMP), have been obtained using radiolysis experiments and computational methods. The bimolecular rate constant for the •OH reaction with DMP was determined to be (3.2 ± 0.1) × 109 M–1s–1. The possible reaction mechanisms of radical adduct formation (RAF), hydrogen atom transfer (HAT), and single electron transfer (SET) were considered. By comparing the experimental absorption spectra with the computational results, it was concluded that the RAF and HAT were the dominant reaction pathways, and OH-adducts (•DMPOH1, •DMPOH2) and methyl type radicals •DMP(-H)α were identified as dominated intermediates. Computational results confirmed the identification of transient species with maximum absorption around 260 nm as •DMPOH1 and •DMP(-H)α, and these radical intermediates then converted to monohydroxylated dimethyl phthalates and monomethyl phthalates. Experimental and computational analyses which elucidated the mechanism of •OH-mediated degradation of DMP are discussed in detail.
450. Excited State Behavior of Luminescent Glutathione Protected Gold Clusters Stamplecoskie, K. G.; Chen, Y.-S.; Kamat, P. V. J. Phys. Chem. C 2014, 118 (2), 1370-1376.
 The excited state behavior of luminescent gold clusters provide new insights in understanding their photocatalytic activity in the visible region. The excited state of glutathione protected gold nanoclusters (AuGSH) which is characterized by the long-lived excited state (τ = 780 ns) arises from the ligand-to-metal type transition. These AuGSH clusters are in a partially oxidized state, (Au(I))and are readily reduced by chemical or electrochemical methods. Interestingly a metal core transition with short lived lifetime (τ <3 ps) appears along with a longer lifetime in reduced AuGSH clusters. The role of oxidation state of gold clusters in dictating the photocatalytic reduction of methyl viologen is discussed.
The excited state behavior of luminescent gold clusters provide new insights in understanding their photocatalytic activity in the visible region. The excited state of glutathione protected gold nanoclusters (AuGSH) which is characterized by the long-lived excited state (τ = 780 ns) arises from the ligand-to-metal type transition. These AuGSH clusters are in a partially oxidized state, (Au(I))and are readily reduced by chemical or electrochemical methods. Interestingly a metal core transition with short lived lifetime (τ <3 ps) appears along with a longer lifetime in reduced AuGSH clusters. The role of oxidation state of gold clusters in dictating the photocatalytic reduction of methyl viologen is discussed.
449. An Inorganic Hole Conductor for Organo-Lead Halide Perovskite Solar Cells. Improved Hole Conductivity with Copper Iodide Christians, J. A.; Fung, R. C. A.; Kamat, P. V. J. Am. Chem. Soc. 2014, 136 (2), 758-764.
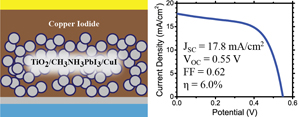 Organo-lead halide perovskite solar cells have emerged as one of the most promising candidates for the next generation of solar cells. To date, these perovskite thin film solar cells have exclusively employed organic hole conducting polymers which are often expensive and have low hole mobility. In a quest to explore new inorganic hole conducting materials for these perovskite based thin film photovoltaics, we have identified copper iodide as a possible alternative. Using copper iodide, we have succeeded in achieving a promising power conversion efficiency of 6.0% with excellent photocurrent stability. The open-circuit voltage, compared to the best spiro-OMeTAD devices, remains low and is attributed to higher recombination in CuI devices as determined by impedance spectroscopy. However, impedance spectroscopy revealed that CuI exhibits two orders of magnitude higher electrical conductivity than spiro-OMeTAD which allows for significantly higher fill factors. Reducing the recombination in these devices could render CuI as a cost effective competitor to spiro-OMeTAD in perovskite solar cells.
Organo-lead halide perovskite solar cells have emerged as one of the most promising candidates for the next generation of solar cells. To date, these perovskite thin film solar cells have exclusively employed organic hole conducting polymers which are often expensive and have low hole mobility. In a quest to explore new inorganic hole conducting materials for these perovskite based thin film photovoltaics, we have identified copper iodide as a possible alternative. Using copper iodide, we have succeeded in achieving a promising power conversion efficiency of 6.0% with excellent photocurrent stability. The open-circuit voltage, compared to the best spiro-OMeTAD devices, remains low and is attributed to higher recombination in CuI devices as determined by impedance spectroscopy. However, impedance spectroscopy revealed that CuI exhibits two orders of magnitude higher electrical conductivity than spiro-OMeTAD which allows for significantly higher fill factors. Reducing the recombination in these devices could render CuI as a cost effective competitor to spiro-OMeTAD in perovskite solar cells.
2013
448. Direct Observation of Spatially Heterogeneous Single-Layer Graphene Oxide Reduction Kinetics McDonald, M. P.; Eltom, A.; Vietmeyer, F.; Thapa, J.; Morozov, Y. V.; Sokolov, D. A.; Hodak, J. H.; Vinodgopal, K.; Kamat, P. V.; Kuno, M. Nano Lett. 2013, 13 (12), 5777-5784.
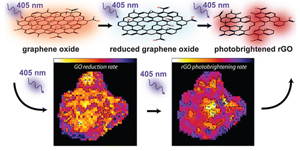 Graphene oxide (GO) is an important precursor in the production of chemically derived graphene. During reduction, GO's electrical conductivity and band gap change gradually. Doping and chemical functionalization are also possible, illustrating GO's immense potential in creating functional devices through control of its local hybridization. Here we show that laser-induced photolysis controllably reduces individual single-layer GO sheets. The reaction can be followed in real time through sizable decreases in GO's photoluminescence efficiency along with spectral blueshifts. As-produced reduced graphene oxide (rGO) sheets undergo additional photolysis, characterized by dramatic emission enhancements and spectral redshifts. Both GO's reduction and subsequent conversion to photobrightened rGO are captured through movies of their photoluminescence kinetics. Rate maps illustrate sizable spatial and temporal heterogeneities in sp2 domain growth and reveal how reduction "flows" across GO and rGO sheets. The observed heterogeneous reduction kinetics provides mechanistic insight into GO's conversion to chemically derived graphene and highlights opportunities for overcoming its dynamic, chemical disorder.
Graphene oxide (GO) is an important precursor in the production of chemically derived graphene. During reduction, GO's electrical conductivity and band gap change gradually. Doping and chemical functionalization are also possible, illustrating GO's immense potential in creating functional devices through control of its local hybridization. Here we show that laser-induced photolysis controllably reduces individual single-layer GO sheets. The reaction can be followed in real time through sizable decreases in GO's photoluminescence efficiency along with spectral blueshifts. As-produced reduced graphene oxide (rGO) sheets undergo additional photolysis, characterized by dramatic emission enhancements and spectral redshifts. Both GO's reduction and subsequent conversion to photobrightened rGO are captured through movies of their photoluminescence kinetics. Rate maps illustrate sizable spatial and temporal heterogeneities in sp2 domain growth and reveal how reduction "flows" across GO and rGO sheets. The observed heterogeneous reduction kinetics provides mechanistic insight into GO's conversion to chemically derived graphene and highlights opportunities for overcoming its dynamic, chemical disorder.
447. Sequentially Layered CdSe/CdS Nanowire Architecture for Improved Nanowire Solar Cell Performance Choi, H.; Radich, J. G.; Kamat, P. V. J. Phys. Chem. C 2013, 118 (1), 206–213.
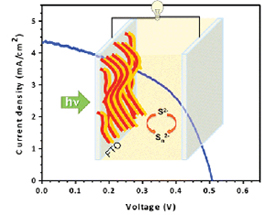 The power conversion efficiency of semiconductor nanowire (NW) based solar cells as compared to quantum dot solar cell (QDSC) has remained lower, and efforts to improve the photovoltaic performance of semiconductor NWs continue. We have now succeeded in using a layered architecture of CdS and CdSe NWs for improving the photovoltaic performance of nanowire solar cell (NWSC). The photoanode designed with sequentially deposited films of CdSe and CdS NWs delivered a power conversion efficiency of 1%. This efficiency of CdSe/CdS composite is an order of magnitude improvement over single nanowire system (CdS or CdSe) based solar cell. The improvement seen in the CdSe/CdS composite film is attributed to charge rectification and improvement of electron and hole separation and transport in the opposite direction. Impedance spectroscopy demonstrates the beneficial effect of type II structure in CdSe/CdS sequential deposition through lower transport resistance, which remains a dominating effect in dictating the overall performance of the NWSC.
The power conversion efficiency of semiconductor nanowire (NW) based solar cells as compared to quantum dot solar cell (QDSC) has remained lower, and efforts to improve the photovoltaic performance of semiconductor NWs continue. We have now succeeded in using a layered architecture of CdS and CdSe NWs for improving the photovoltaic performance of nanowire solar cell (NWSC). The photoanode designed with sequentially deposited films of CdSe and CdS NWs delivered a power conversion efficiency of 1%. This efficiency of CdSe/CdS composite is an order of magnitude improvement over single nanowire system (CdS or CdSe) based solar cell. The improvement seen in the CdSe/CdS composite film is attributed to charge rectification and improvement of electron and hole separation and transport in the opposite direction. Impedance spectroscopy demonstrates the beneficial effect of type II structure in CdSe/CdS sequential deposition through lower transport resistance, which remains a dominating effect in dictating the overall performance of the NWSC.
446. CdS Nanowire Solar Cells: Dual Role of Squaraine Dye as a Sensitizer and a Hole Transporter Choi, H.; Kamat, P. V. J. Phys. Chem. Lett. 2013, 4, 3983–3991.
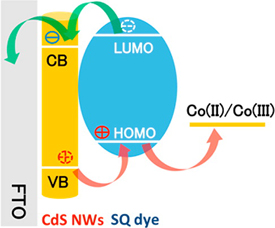 The squaraine dye (SQ) anchored onto CdS nanowires serves as a photosensitizing dye and a hole acceptor. This dual role of the squaraine dye has been successfully exploited in a nanowire solar cell to improve the photoconversion efficiency. Electrophoretic deposition of CdS NWs and CdS NWs+SQ composite onto conducting glass electrodes was performed to obtain robust photoanodes and evaluate the photovoltaic performance of nanowire solar cells (NWSCs). Whereas the sensitization property of the SQ extends the response of CdS NWSCs into the near-IR (NIR) region, its redox property facilitates shuttling of holes to the electrolyte and suppressing the charge recombination process. Transient absorption measurements confirm the formation of cation radical of the dye arising from these two processes. The dual role of the squaraine dye has enabled us to improve the power conversion efficiency of NWSCs by a factor of ~20. Photoelectrochemical, spectroelectrochemical, and spectroscopic measurements provide insight into the multifaceted role of squaraine dye in improving the performance of NWSCs.
The squaraine dye (SQ) anchored onto CdS nanowires serves as a photosensitizing dye and a hole acceptor. This dual role of the squaraine dye has been successfully exploited in a nanowire solar cell to improve the photoconversion efficiency. Electrophoretic deposition of CdS NWs and CdS NWs+SQ composite onto conducting glass electrodes was performed to obtain robust photoanodes and evaluate the photovoltaic performance of nanowire solar cells (NWSCs). Whereas the sensitization property of the SQ extends the response of CdS NWSCs into the near-IR (NIR) region, its redox property facilitates shuttling of holes to the electrolyte and suppressing the charge recombination process. Transient absorption measurements confirm the formation of cation radical of the dye arising from these two processes. The dual role of the squaraine dye has enabled us to improve the power conversion efficiency of NWSCs by a factor of ~20. Photoelectrochemical, spectroelectrochemical, and spectroscopic measurements provide insight into the multifaceted role of squaraine dye in improving the performance of NWSCs.
445. Nickel-Doped MnO2 Nanowires Anchored onto Reduced Graphene Oxide for Rapid Cycling Cathode in Lithium Ion Batteries Radich, J. G.; Chen, Y.-S.; Kamat, P. V. ECS Journal of Solid State Science and Technology 2013, 2 (10), M3178-M3181.
 Nickel-doped MnO2 nanowires were synthesized directly onto reduced graphene oxide (RGO) to generate a composite cathode material with improved high-rate cycling characteristics. The presence of RGO improves the electrochemical characteristics of the cathode in Li-ion half-cell architecture. Cyclic voltammetry, electrochemical impedance spectroscopy, and electrode cycling are confirm that RGO plays a major role in enhancing the ability of the NixMn(1-x)O2 to reversibly intercalate lithium ions at 1C rate. The chronocoulometric response of the RGO-based electrode shows the improvements originate from faster reaction kinetics and transport of Li+ coupled with increased specific capacitance and Li+ adsorption.
Nickel-doped MnO2 nanowires were synthesized directly onto reduced graphene oxide (RGO) to generate a composite cathode material with improved high-rate cycling characteristics. The presence of RGO improves the electrochemical characteristics of the cathode in Li-ion half-cell architecture. Cyclic voltammetry, electrochemical impedance spectroscopy, and electrode cycling are confirm that RGO plays a major role in enhancing the ability of the NixMn(1-x)O2 to reversibly intercalate lithium ions at 1C rate. The chronocoulometric response of the RGO-based electrode shows the improvements originate from faster reaction kinetics and transport of Li+ coupled with increased specific capacitance and Li+ adsorption.
444. Trap and Transfer. Two-Step Hole Injection Across the Sb2S3/CuSCN Interface in Solid State Solar Cells. Christians, J. A.; Kamat, P. V. ACS Nano 2013, 7 (9), 7967–7974.
 In solid-state semiconductor-sensitized solar cells, commonly known as extremely thin absorber (ETA) or solid-state quantum dot sensitized solar cells (QDSCs), transfer of photogenerated holes from the absorber species to the p-type hole conductor plays a critical role in the charge separation process. Using Sb2S3 (absorber) and CuSCN (hole conductor), we have constructed ETA solar cells exhibiting a power conversion efficiency of 3.3%. The hole transfer from excited Sb2S3 into CuSCN, which limits the overall power conversion efficiency of these solar cells, is now independently studied using transient absorption spectroscopy. In the Sb2S3 absorber layer, photogenerated holes are rapidly localized on the sulfur atoms of the crystal lattice, forming a sulfide radical (S−•) species. This trapped hole is transferred from the Sb2S3 absorber to the CuSCN hole conductor with an exponential time constant of 1680 ps. This process was monitored through the spectroscopic signal seen for the S−• species in Sb2S3, providing direct evidence for the hole transfer dynamics in ETA solar cells. Elucidation of the hole transfer mechanism from Sb2S3 to CuSCN represents a significant step toward understanding charge separation in Sb2S3 solar cells, and provides insight into the design of new architectures for higher efficiency devices.
In solid-state semiconductor-sensitized solar cells, commonly known as extremely thin absorber (ETA) or solid-state quantum dot sensitized solar cells (QDSCs), transfer of photogenerated holes from the absorber species to the p-type hole conductor plays a critical role in the charge separation process. Using Sb2S3 (absorber) and CuSCN (hole conductor), we have constructed ETA solar cells exhibiting a power conversion efficiency of 3.3%. The hole transfer from excited Sb2S3 into CuSCN, which limits the overall power conversion efficiency of these solar cells, is now independently studied using transient absorption spectroscopy. In the Sb2S3 absorber layer, photogenerated holes are rapidly localized on the sulfur atoms of the crystal lattice, forming a sulfide radical (S−•) species. This trapped hole is transferred from the Sb2S3 absorber to the CuSCN hole conductor with an exponential time constant of 1680 ps. This process was monitored through the spectroscopic signal seen for the S−• species in Sb2S3, providing direct evidence for the hole transfer dynamics in ETA solar cells. Elucidation of the hole transfer mechanism from Sb2S3 to CuSCN represents a significant step toward understanding charge separation in Sb2S3 solar cells, and provides insight into the design of new architectures for higher efficiency devices.
443. Quantum Dot Surface Chemistry: Ligand Effects and Electron Transfer Reactions. Hines, D. A.; Kamat, P. V. J. Phys. Chem. C 2013, 117 (27), 14418–14426.
 With the increased interest in quantum dot sensitized solar cells (QDSCs) there comes a need to better understand how surface modification of quantum dots (QDs) can affect the excited state dynamics of QDs, electron transfer at the QD-metal oxide (MO) interface, and overall photoconversion efficiency of QDSCs. We have monitored the surface modification of solution based QDs via the steady-state absorption and emission characteristics of colloidal CdSe passivated with β-Alanine (β-Ala). The trap-remediating nature of the β-Ala molecule, arising from the Lewis-basicity of the amine group, is realized from the hypsochromic shifts seen in excitonic absorption and emission bands as well as an increase in fluorescence quantum yield. Transient absorption measurements of CdSe-TiO2 films prepared with and without β-Ala as a linker molecule further reveal the role of the surface modifier in influencing excited state electron transfer processes. Electron transfer at this interface was dependent on the method of QD deposition: CdSe-TiO2 (direct deposition, ket = 1.5 × 10^10 s-1), CdSe-linker-TiO2 (attaching linker molecule first to TiO2 so that β-Ala interaction is minimal, ket = 2.4 × 10^9 s-1) or linker-CdSe-linker-TiO2 (linkage via full β-Ala encapsulation in solution prior to deposition, ket = 6.4 × 10^8 s-1). These results imply that the surface chemistry of colloidal CdSe plays an important role in mediating electron transfer reactions.
With the increased interest in quantum dot sensitized solar cells (QDSCs) there comes a need to better understand how surface modification of quantum dots (QDs) can affect the excited state dynamics of QDs, electron transfer at the QD-metal oxide (MO) interface, and overall photoconversion efficiency of QDSCs. We have monitored the surface modification of solution based QDs via the steady-state absorption and emission characteristics of colloidal CdSe passivated with β-Alanine (β-Ala). The trap-remediating nature of the β-Ala molecule, arising from the Lewis-basicity of the amine group, is realized from the hypsochromic shifts seen in excitonic absorption and emission bands as well as an increase in fluorescence quantum yield. Transient absorption measurements of CdSe-TiO2 films prepared with and without β-Ala as a linker molecule further reveal the role of the surface modifier in influencing excited state electron transfer processes. Electron transfer at this interface was dependent on the method of QD deposition: CdSe-TiO2 (direct deposition, ket = 1.5 × 10^10 s-1), CdSe-linker-TiO2 (attaching linker molecule first to TiO2 so that β-Ala interaction is minimal, ket = 2.4 × 10^9 s-1) or linker-CdSe-linker-TiO2 (linkage via full β-Ala encapsulation in solution prior to deposition, ket = 6.4 × 10^8 s-1). These results imply that the surface chemistry of colloidal CdSe plays an important role in mediating electron transfer reactions.
442. Metal-Cluster-Sensitized Solar Cells. A New Class of Thiolated Gold Sensitizers Delivering Efficiency Greater Than 2%. Chen, Y.-S.; Choi, H.; Kamat, P. V. J. Am. Chem. Soc. 2013, 135 (24), pp 8822–8825.
 A new class of metal-cluster sensitizers has been explored for designing high-efficiency solar cells. Thiol-protected gold clusters which exhibit molecular-like properties have been found to inject electrons into TiO2 nanostructures under visible excitation. Mesoscopic TiO2 films modified with gold clusters deliver stable photocurrent of 3.96 mA/cm2 with power conversion efficiencies of 2.3% under AM 1.5 illumination. The overall absorption features and cell performance of metal-cluster-sensitized solar cells (MCSCs) are comparable to those of CdS quantum-dot-based solar cells (QDSCs). The relatively high open-circuit voltage of 832 mV and fill factor of 0.7 for MCSCs as compared to QDSCs show the viability of these new sensitizers as alternatives to semiconductor QDs and sensitizing dyes in the next generation of solar cells. The superior performance of MCSCs discussed in this maiden study lays the foundation to explore other metal clusters with broader visible absorption.
A new class of metal-cluster sensitizers has been explored for designing high-efficiency solar cells. Thiol-protected gold clusters which exhibit molecular-like properties have been found to inject electrons into TiO2 nanostructures under visible excitation. Mesoscopic TiO2 films modified with gold clusters deliver stable photocurrent of 3.96 mA/cm2 with power conversion efficiencies of 2.3% under AM 1.5 illumination. The overall absorption features and cell performance of metal-cluster-sensitized solar cells (MCSCs) are comparable to those of CdS quantum-dot-based solar cells (QDSCs). The relatively high open-circuit voltage of 832 mV and fill factor of 0.7 for MCSCs as compared to QDSCs show the viability of these new sensitizers as alternatives to semiconductor QDs and sensitizing dyes in the next generation of solar cells. The superior performance of MCSCs discussed in this maiden study lays the foundation to explore other metal clusters with broader visible absorption.
441. Making Graphene Holey. Gold-Nanoparticle-Mediated Hydroxyl Radical Attack on Reduced Graphene Oxide Radich, J. G.; Kamat, P. V. ACS Nano 2013, 7 (6), 5546–5557.
 Graphene oxide (GO) and reduced graphene oxide (RGO) have important applications in the development of new electrode and photocatalyst architectures. Gold nanoparticles (AuNPs) have now been employed as catalyst to generate OH• and oxidize RGO via hydroxyl radical attack. The oxidation of RGO is marked by pores and wrinkles within the 2-D network. Nanosecond laser flash photolysis was used in conjunction with competition kinetics to elucidate the oxidative mechanism and calculate rate constants for the AuNP-catalyzed and direct reaction between RGO and OH•. The results highlight the use of the AuNP-mediated oxidation reaction to tune the properties of RGO through the degree of oxidation and/or functional group selectivity in addition to the nanoporous and wrinkle facets. The ability of AuNPs to catalyze the photolytic decomposition of H2O2 as well as the hydroxyl radical-induced oxidation of RGO raises new issues concerning graphene stability in energy conversion and storage (photocatalysis, fuel cells, Li-ion batteries, etc.). Understanding RGO oxidation by free radicals will aid in maintaining the long-term stability of RGO-based functional composites where intimate contact with radical species is inevitable.
Graphene oxide (GO) and reduced graphene oxide (RGO) have important applications in the development of new electrode and photocatalyst architectures. Gold nanoparticles (AuNPs) have now been employed as catalyst to generate OH• and oxidize RGO via hydroxyl radical attack. The oxidation of RGO is marked by pores and wrinkles within the 2-D network. Nanosecond laser flash photolysis was used in conjunction with competition kinetics to elucidate the oxidative mechanism and calculate rate constants for the AuNP-catalyzed and direct reaction between RGO and OH•. The results highlight the use of the AuNP-mediated oxidation reaction to tune the properties of RGO through the degree of oxidation and/or functional group selectivity in addition to the nanoporous and wrinkle facets. The ability of AuNPs to catalyze the photolytic decomposition of H2O2 as well as the hydroxyl radical-induced oxidation of RGO raises new issues concerning graphene stability in energy conversion and storage (photocatalysis, fuel cells, Li-ion batteries, etc.). Understanding RGO oxidation by free radicals will aid in maintaining the long-term stability of RGO-based functional composites where intimate contact with radical species is inevitable.
440. Photoactive Porous Silicon Nanopowder. Meekins, B. H.; Lin, Y. C.; Manser, J. S.; Manukyan, K.; Mukasyan, A. S.; Kamat, P. V.; McGinn, P. J. ACS Appl. Mater. Interfaces 2013, 5 (8), 2943–2951.
 Bulk processing of porous silicon nanoparticles (nSi) of 50–300 nm size and surface area of 25–230 m2/g has been developed using a combustion synthesis method. nSi exhibits consistent photoresponse to AM 1.5 simulated solar excitation. In confirmation of photoactivity, the films of nSi exhibit prompt bleaching following femtosecond laser pulse excitation resulting from the photoinduced charge separation. Photocurrent generation observed upon AM 1.5 excitation of these films in a photoelectrochemical cell shows strong dependence on the thickness of the intrinsic silica shell that encompasses the nanoparticles and hinders interparticle electron transfer.
Bulk processing of porous silicon nanoparticles (nSi) of 50–300 nm size and surface area of 25–230 m2/g has been developed using a combustion synthesis method. nSi exhibits consistent photoresponse to AM 1.5 simulated solar excitation. In confirmation of photoactivity, the films of nSi exhibit prompt bleaching following femtosecond laser pulse excitation resulting from the photoinduced charge separation. Photocurrent generation observed upon AM 1.5 excitation of these films in a photoelectrochemical cell shows strong dependence on the thickness of the intrinsic silica shell that encompasses the nanoparticles and hinders interparticle electron transfer.
439. CuInS2-Sensitized Quantum Dot Solar Cell. Electrophoretic Deposition, Excited-State Dynamics, and Photovoltaic Performance. Santra, P. K.; Nair, P. V.; Thomas, K. G.; Kamat, P. V. J. Phys. Chem. Lett. 2013, 4, 722–729.
 Ternary metal chalcogenides such as CuInS2 offer new opportunities to design quantum dot solar cells (QDSC). Chemically synthesized CuInS2 quantum dots (particle diameter, 2.6 nm) have been successfully deposited within the mesoscopic TiO2 film using electrophoretic deposition (150 V cm–1 dc field). The primary photoinduced process of electron injection from excited CuInS2 into TiO2 occurs with a rate constant of 5.75 × 1011 s–1. The TiO2/CuInS2 films are photoactive and produce anodic photocurrent with a power conversion efficiency of 1.14%. Capping the TiO2/CuInS2 film with a CdS layer decreases the interfacial charge recombination and thus offers further improvement in the power conversion efficiency (3.91%). The synergy of using CdS as a passivation layer in the composite film is also evident from the increased external quantum efficiency of the electrode in the red region where only CuInS2 absorbs the incident light.
Ternary metal chalcogenides such as CuInS2 offer new opportunities to design quantum dot solar cells (QDSC). Chemically synthesized CuInS2 quantum dots (particle diameter, 2.6 nm) have been successfully deposited within the mesoscopic TiO2 film using electrophoretic deposition (150 V cm–1 dc field). The primary photoinduced process of electron injection from excited CuInS2 into TiO2 occurs with a rate constant of 5.75 × 1011 s–1. The TiO2/CuInS2 films are photoactive and produce anodic photocurrent with a power conversion efficiency of 1.14%. Capping the TiO2/CuInS2 film with a CdS layer decreases the interfacial charge recombination and thus offers further improvement in the power conversion efficiency (3.91%). The synergy of using CdS as a passivation layer in the composite film is also evident from the increased external quantum efficiency of the electrode in the red region where only CuInS2 absorbs the incident light.
438. Quantum Dot Solar Cells. The Next Big Thing in Photovoltaics. Kamat, P. V. J. Phys. Chem. Lett. 2013, 4, 908–918.
 The recent surge in the utilization of semiconductor nanostructures for solar energy conversion has led to the development of high-efficiency solar cells. Some of these recent advances are in the areas of synthesis of new semiconductor materials and the ability to tune the electronic properties through size, shape, and composition and to assemble quantum dots as hybrid assemblies. In addition, processes such as hot electron injection, multiple exciton generation (MEG), plasmonic effects, and energy-transfer-coupled electron transfer are gaining momentum to overcome the efficiency limitations of energy capture and conversion. The recent advances as well as future prospects of quantum dot solar cells discussed in this perspective provide the basis for consideration as "The Next Big Thing" in photovoltaics.
The recent surge in the utilization of semiconductor nanostructures for solar energy conversion has led to the development of high-efficiency solar cells. Some of these recent advances are in the areas of synthesis of new semiconductor materials and the ability to tune the electronic properties through size, shape, and composition and to assemble quantum dots as hybrid assemblies. In addition, processes such as hot electron injection, multiple exciton generation (MEG), plasmonic effects, and energy-transfer-coupled electron transfer are gaining momentum to overcome the efficiency limitations of energy capture and conversion. The recent advances as well as future prospects of quantum dot solar cells discussed in this perspective provide the basis for consideration as "The Next Big Thing" in photovoltaics.
437. CdSe Nanowire Solar Cells Using Carbazole as a Surface Modifier. Choi, H.; Kuno, M.; Hartland, G. V.; Kamat, P. V. J. Mater. Chem. A 2013, 1, 5487-5491.
 Carbazole molecules containing thiol functional groups, when attached to CdSe nanowires (NWs), facilitate hole transport across semiconductor interfaces. The improved hole transfer rate is evidenced by increased electron lifetimes and better photovoltaic performance. Nanowire solar cells (NWSCs) with carbazole treatment delivered a power conversion efficiency of 0.46%, which is an order of magnitude improvement over untreated films. The illumination of the sample during the electrophoretic deposition of nanowires also had a profound effect in obtaining stable and higher photocurrents.
Carbazole molecules containing thiol functional groups, when attached to CdSe nanowires (NWs), facilitate hole transport across semiconductor interfaces. The improved hole transfer rate is evidenced by increased electron lifetimes and better photovoltaic performance. Nanowire solar cells (NWSCs) with carbazole treatment delivered a power conversion efficiency of 0.46%, which is an order of magnitude improvement over untreated films. The illumination of the sample during the electrophoretic deposition of nanowires also had a profound effect in obtaining stable and higher photocurrents.
436. Reduced Graphene Oxide–Silver Nanoparticle Composite as an Active SERS Material. Murphy, S.; Huang, L.; Kamat, P. V. J. Phys. Chem. C 2013, 117 (9), 4740–4747.
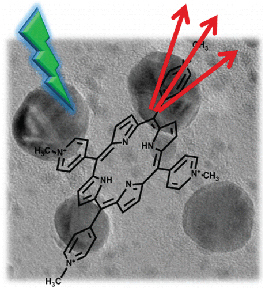 Selectivity and enhanced sensitivity for SERS measurements are highly desirable for environmental and analytical applications. Interaction of a target molecule with SERS substrate plays a pivotal role in determining the magnitude of enhancement and spectral profile of the SERS signal. A reduced graphene oxide–Ag nanoparticle (RGO-Ag NP) composite has been designed to boost SERRS sensitivity of a porphyrin derivative. Complexation between 5,10,15,20-tetrakis(1-methyl-4-pyridinio)porphyrin tetra(p-toluenesulfonate) (TMPyP) porphyrin and the RGO-Ag NP composite is evidenced by a red-shifted porphyrin absorption band. Results indicate complexation is influential in improved surface-enhanced resonance Raman (SERRS) signal for TMPyP and thus offers an advantage for target molecule detection at low concentration levels. The combined effects of RGO and Ag NPs in the enhancement of SERS signal of TMPyP are discussed.
Selectivity and enhanced sensitivity for SERS measurements are highly desirable for environmental and analytical applications. Interaction of a target molecule with SERS substrate plays a pivotal role in determining the magnitude of enhancement and spectral profile of the SERS signal. A reduced graphene oxide–Ag nanoparticle (RGO-Ag NP) composite has been designed to boost SERRS sensitivity of a porphyrin derivative. Complexation between 5,10,15,20-tetrakis(1-methyl-4-pyridinio)porphyrin tetra(p-toluenesulfonate) (TMPyP) porphyrin and the RGO-Ag NP composite is evidenced by a red-shifted porphyrin absorption band. Results indicate complexation is influential in improved surface-enhanced resonance Raman (SERRS) signal for TMPyP and thus offers an advantage for target molecule detection at low concentration levels. The combined effects of RGO and Ag NPs in the enhancement of SERS signal of TMPyP are discussed.
435. Tandem-Layered Quantum Dot Solar Cells: Tuning the Photovoltaic Response with Luminescent Ternary Cadmium Chalcogenides. Santra, P.; Kamat, P. V. J. Am. Chem. Soc. 2013, 135 (2), 877–885.
 Photon management in solar cells is an important criterion as it enables the capture of incident visible and infrared photons in an efficient way. Highly luminescent CdSeS quantum dots (QDs) with a diameter of 4.5 nm were prepared with a gradient structure that allows tuning of absorption and emission bands over the entire visible region without varying the particle size. These crystalline ternary cadmium chalcogenides were deposited within a mesoscopic TiO2 film by electrophoretic deposition with a sequentially-layered architecture. This approach enabled us to design tandem layers of CdSeS QDs of varying band gap within the photoactive anode of a QD solar cell (QDSC). An increase in power conversion efficiency of 1.97–2.81% with decreasing band gap was observed for single-layer CdSeS, thus indicating varying degrees of photon harvesting. In two- and three-layered tandem QDSCs, we observed maximum power conversion efficiencies of 3.2 and 3.0%, respectively. These efficiencies are greater than the values obtained for the three individually layered photoanodes. The synergy of using tandem layers of the ternary semiconductor CdSeS in QDSCs was systematically evaluated using transient spectroscopy and photoelectrochemistry.
Photon management in solar cells is an important criterion as it enables the capture of incident visible and infrared photons in an efficient way. Highly luminescent CdSeS quantum dots (QDs) with a diameter of 4.5 nm were prepared with a gradient structure that allows tuning of absorption and emission bands over the entire visible region without varying the particle size. These crystalline ternary cadmium chalcogenides were deposited within a mesoscopic TiO2 film by electrophoretic deposition with a sequentially-layered architecture. This approach enabled us to design tandem layers of CdSeS QDs of varying band gap within the photoactive anode of a QD solar cell (QDSC). An increase in power conversion efficiency of 1.97–2.81% with decreasing band gap was observed for single-layer CdSeS, thus indicating varying degrees of photon harvesting. In two- and three-layered tandem QDSCs, we observed maximum power conversion efficiencies of 3.2 and 3.0%, respectively. These efficiencies are greater than the values obtained for the three individually layered photoanodes. The synergy of using tandem layers of the ternary semiconductor CdSeS in QDSCs was systematically evaluated using transient spectroscopy and photoelectrochemistry.
434. Galvanic Exchange on Reduced Graphene Oxide. Designing a Multifunctional Two-Dimensional Catalyst Assembly. Krishnamurthy, S.; Kamat, P. V. J. Phys. Chem. C 2013, 117 (1), 571–577.
 The two-dimensional network of reduced graphene oxide (RGO) is decorated with silver and gold nanoparticles. The silver nanoparticles deposited on RGO by photocatalytic reduction are subjected to galvanic exchange with Au3+ ions to transform them into gold nanoparticles. This compositional change on the RGO surface demonstrates RGO's versatile ability to anchor a wide array of nano-particles and facilitate chemical transformations. Coupled with RGO's unique ability to capture and transport electrons, galvanic exchange is used to contrive a two-dimensional nano catalyst mat. Raman studies show that metal nanoparticles anchored on reduced graphene oxide facilitate enhancement of Raman bands. Using methyl viologen as a probe we elucidate the photocatalytic activity of the Semiconductor-RGO-Metal nanoassembly and highlight the mediation of RGO in charge transfer processes.
The two-dimensional network of reduced graphene oxide (RGO) is decorated with silver and gold nanoparticles. The silver nanoparticles deposited on RGO by photocatalytic reduction are subjected to galvanic exchange with Au3+ ions to transform them into gold nanoparticles. This compositional change on the RGO surface demonstrates RGO's versatile ability to anchor a wide array of nano-particles and facilitate chemical transformations. Coupled with RGO's unique ability to capture and transport electrons, galvanic exchange is used to contrive a two-dimensional nano catalyst mat. Raman studies show that metal nanoparticles anchored on reduced graphene oxide facilitate enhancement of Raman bands. Using methyl viologen as a probe we elucidate the photocatalytic activity of the Semiconductor-RGO-Metal nanoassembly and highlight the mediation of RGO in charge transfer processes.
433. Graphitic Design: Prospects of Graphene-Based Nanocomposites for Solar Energy Conversion, Storage, and Sensing. Lightcap, I. V.; Kamat, P. V. Acc. Chem. Res. 2013, 46 (10), 2235–2243.
 Graphene not only possesses interesting electrochemical behavior but also has a remarkable surface area and mechanical strength and is naturally abundant, all advantageous properties for the design of tailored composite materials. Graphene–semiconductor or −metal nanoparticle composites have the potential to function as efficient, multifunctional materials for energy conversion and storage. These next-generation composite systems could possess the capability to integrate conversion and storage of solar energy, detection, and selective destruction of trace environmental contaminants or achieve single-substrate, multistep heterogeneous catalysis. These advanced materials may soon become a reality, based on encouraging results in the key areas of energy conversion and sensing using graphene oxide as a support structure. Through recent advances, chemists can now integrate such processes on a single substrate while using synthetic designs that combine simplicity with a high degree of structural and composition selectivity. This progress represents the beginning of a transformative movement leveraging the advancements of single-purpose chemistry toward the creation of composites designed to address whole-process applications.
Graphene not only possesses interesting electrochemical behavior but also has a remarkable surface area and mechanical strength and is naturally abundant, all advantageous properties for the design of tailored composite materials. Graphene–semiconductor or −metal nanoparticle composites have the potential to function as efficient, multifunctional materials for energy conversion and storage. These next-generation composite systems could possess the capability to integrate conversion and storage of solar energy, detection, and selective destruction of trace environmental contaminants or achieve single-substrate, multistep heterogeneous catalysis. These advanced materials may soon become a reality, based on encouraging results in the key areas of energy conversion and sensing using graphene oxide as a support structure. Through recent advances, chemists can now integrate such processes on a single substrate while using synthetic designs that combine simplicity with a high degree of structural and composition selectivity. This progress represents the beginning of a transformative movement leveraging the advancements of single-purpose chemistry toward the creation of composites designed to address whole-process applications.
The promising field of graphene nanocomposites for sensing and energy applications is based on fundamental studies that explain the electronic interactions between semiconductor or metal nanoparticles and graphene. In particular, reduced graphene oxide is a suitable composite substrate because of its two-dimensional structure, outstanding surface area, and electrical conductivity. In this Account, we describe common assembly methods for graphene composite materials and examine key studies that characterize its excited state interactions. We also discuss strategies to develop graphene composites and control electron capture and transport through the 2D carbon network. In addition, we provide a brief overview of advances in sensing, energy conversion, and storage applications that incorporate graphene-based composites. With these results in mind, we can envision a new class of semiconductor– or metal–graphene composites sensibly tailored to address the pressing need for advanced energy conversion and storage devices.
432. Photoinduced Charging and Discharging of ZnO Nanoparticles on Graphene Oxide Sheets Yokomizo, Y.; Krishnamurthy, S.; Kamat, P. V.Catalysis Today 2013, 199, 36-41.
 Graphene oxide (GO) serves as a two-dimensional carbon nano-mat to anchor catalyst nanoparticles. We have developed a photocatalyst assembly by anchoring ZnO and Ag nanoparticles on graphene oxide sheets suspended in ethanol. Upon photoirradiation, the electrons are transferred from ZnO to GO to produce reduced graphene oxide (RGO). The ZnO–RGO composites are further decorated with Ag nanoparticles by reducing Ag+ ions quantitatively with excess electrons stored in RGO. Under continuous UV-illumination we observe charging of ZnO nanoparticles as evidenced by the shift in absorption edge. However, no shift in the band edge is seen for ZnO–RGO or ZnO–RGO–Ag composites under UV irradiation indicating the quick discharge of electrons on RGO surface. Such charge–discharge phenomenon on the graphene oxide sheet was further probed by carrying out reduction of methyl viologen. Improved charge separation and selectivity in the reduction process was achieved in these graphene based photocatalytic assemblies.
Graphene oxide (GO) serves as a two-dimensional carbon nano-mat to anchor catalyst nanoparticles. We have developed a photocatalyst assembly by anchoring ZnO and Ag nanoparticles on graphene oxide sheets suspended in ethanol. Upon photoirradiation, the electrons are transferred from ZnO to GO to produce reduced graphene oxide (RGO). The ZnO–RGO composites are further decorated with Ag nanoparticles by reducing Ag+ ions quantitatively with excess electrons stored in RGO. Under continuous UV-illumination we observe charging of ZnO nanoparticles as evidenced by the shift in absorption edge. However, no shift in the band edge is seen for ZnO–RGO or ZnO–RGO–Ag composites under UV irradiation indicating the quick discharge of electrons on RGO surface. Such charge–discharge phenomenon on the graphene oxide sheet was further probed by carrying out reduction of methyl viologen. Improved charge separation and selectivity in the reduction process was achieved in these graphene based photocatalytic assemblies.
2012

431. Realizing Visible Photoactivity of Metal Nanoparticles: Excited-State Behavior and Electron-Transfer Properties of Silver (Ag8) Clusters. Chen, W. T.; Hsu, Y. J.; Kamat, P. V. J. Phys. Chem. Lett. 2012, 3, 2493–2499.
 Silver nanoclusters complexed with dihydrolipoic acid (DHLA) exhibit molecular-like excited-state properties with well-defined absorption and emission features. The 1.8 nm diameter Ag nanoparticles capped with Ag8 clusters exhibit fluorescence maximum at 660 nm with a quantum yield of 0.07%. Although the excited state is relatively short-lived (τ 130 ps), it exhibits significant photochemical reactivity. By introducing MV2+ as a probe, we have succeeded in elucidating the interfacial electron transfer dynamics of Ag nanoclusters. The formation of MV+• as the electron-transfer product with a rate constant of 2.74 × 1010 s–1 confirms the ability of these metal clusters to participate in the photocatalytic reduction process. Basic understanding of excited-state processes in fluorescent metal clusters paves the way toward the development of biological probes, sensors, and catalysts in energy conversion devices.
Silver nanoclusters complexed with dihydrolipoic acid (DHLA) exhibit molecular-like excited-state properties with well-defined absorption and emission features. The 1.8 nm diameter Ag nanoparticles capped with Ag8 clusters exhibit fluorescence maximum at 660 nm with a quantum yield of 0.07%. Although the excited state is relatively short-lived (τ 130 ps), it exhibits significant photochemical reactivity. By introducing MV2+ as a probe, we have succeeded in elucidating the interfacial electron transfer dynamics of Ag nanoclusters. The formation of MV+• as the electron-transfer product with a rate constant of 2.74 × 1010 s–1 confirms the ability of these metal clusters to participate in the photocatalytic reduction process. Basic understanding of excited-state processes in fluorescent metal clusters paves the way toward the development of biological probes, sensors, and catalysts in energy conversion devices.
430. Dual-Frequency Ultrasound for Designing Two Dimensional Catalyst Surface: Reduced Graphene Oxide-Pt Composite. Vinodgopal, K.; Neppolian, B.; Salleh, N.; Lightcap, I. V.; Grieser, F.; Ashokkumar, M.; Ding, T. T.; Kamat, P. V. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2012, 5, 81-87.
 Few-layered reduced graphene oxide–Pt composites are prepared using a combination of two ultrasound frequencies at 20 kHz and 211 kHz. Such a unique dual frequency arrangement operating in tandem, yields large exfoliated graphene sheets with platinum nanoparticles dispersed on them. The extent of reduction achieved by the use of this dual frequency sonication arrangement is evaluated by XPS, IR, and Raman spectroscopies. Transmission electron and atomic force microscopies confirm the morphology of resulting assemblies to be bi- and single layered sheets. These composites show good electrocatalytic activity towards methanol oxidation.
Few-layered reduced graphene oxide–Pt composites are prepared using a combination of two ultrasound frequencies at 20 kHz and 211 kHz. Such a unique dual frequency arrangement operating in tandem, yields large exfoliated graphene sheets with platinum nanoparticles dispersed on them. The extent of reduction achieved by the use of this dual frequency sonication arrangement is evaluated by XPS, IR, and Raman spectroscopies. Transmission electron and atomic force microscopies confirm the morphology of resulting assemblies to be bi- and single layered sheets. These composites show good electrocatalytic activity towards methanol oxidation.
429. Photoinduced Surface Oxidation and Its Effect on the Exciton Dynamics of CdSe Quantum Dots. Hines, D. A.; Becker, M. A.; Kamat, P. V. J. Phys. Chem. C 2012, 116 (24),13452–13457.
 With increased interest in semiconductor nanoparticles for use in quantum dot solar cells there comes a need to understand the long-term photostability of such materials. Colloidal CdSe quantum dots (QDs) were suspended in toluene and stored in combinations of light/dark and N2/O2 to simulate four possible benchtop storage environments. CdSe QDs stored in a dark, oxygen-free environment were observed to better retain their optical properties over the course of 90 days. The excited state lifetimes, determined through femtosecond transient absorption spectroscopy, of air-equilibrated samples exposed to light exhibit a decrease in average lifetime (0.81 ns) when compared to samples stored in a nitrogen/dark environment (8.3 ns). A photoetching technique commonly used for controlled reduction of QD size was found to induce energetic trap states to CdSe QDs and accelerate the rate of electron–hole recombination. X-ray absorption near edge structure (XANES) analysis confirms surface oxidation, the extent of which is shown to be dependent on the thickness of the ligand shell.
With increased interest in semiconductor nanoparticles for use in quantum dot solar cells there comes a need to understand the long-term photostability of such materials. Colloidal CdSe quantum dots (QDs) were suspended in toluene and stored in combinations of light/dark and N2/O2 to simulate four possible benchtop storage environments. CdSe QDs stored in a dark, oxygen-free environment were observed to better retain their optical properties over the course of 90 days. The excited state lifetimes, determined through femtosecond transient absorption spectroscopy, of air-equilibrated samples exposed to light exhibit a decrease in average lifetime (0.81 ns) when compared to samples stored in a nitrogen/dark environment (8.3 ns). A photoetching technique commonly used for controlled reduction of QD size was found to induce energetic trap states to CdSe QDs and accelerate the rate of electron–hole recombination. X-ray absorption near edge structure (XANES) analysis confirms surface oxidation, the extent of which is shown to be dependent on the thickness of the ligand shell.
428. Know Thy Nano Neighbor. Plasmonic versus Electron Charging Effects of Metal Nanoparticles in Dye Sensitized Solar Cells. Choi, H.; Chen, W. T.; Kamat, P. V. ACS Nano 2012, 6 (5), 4418–4427.
 With increased interest in semiconductor nanoparticles for use in quantum dot solar cells there comes a need to understand the long-term photostability of such materials. Colloidal CdSe quantum dots (QDs) were suspended in toluene and stored in combinations of light/dark and N2/O2 to simulate four possible benchtop storage environments. CdSe QDs stored in a dark, oxygen-free environment were observed to better retain their optical properties over the course of 90 days. The excited state lifetimes, determined through femtosecond transient absorption spectroscopy, of air-equilibrated samples exposed to light exhibit a decrease in average lifetime (0.81 ns) when compared to samples stored in a nitrogen/dark environment (8.3 ns). A photoetching technique commonly used for controlled reduction of QD size was found to induce energetic trap states to CdSe QDs and accelerate the rate of electron–hole recombination. X-ray absorption near edge structure (XANES) analysis confirms surface oxidation, the extent of which is shown to be dependent on the thickness of the ligand shell.
With increased interest in semiconductor nanoparticles for use in quantum dot solar cells there comes a need to understand the long-term photostability of such materials. Colloidal CdSe quantum dots (QDs) were suspended in toluene and stored in combinations of light/dark and N2/O2 to simulate four possible benchtop storage environments. CdSe QDs stored in a dark, oxygen-free environment were observed to better retain their optical properties over the course of 90 days. The excited state lifetimes, determined through femtosecond transient absorption spectroscopy, of air-equilibrated samples exposed to light exhibit a decrease in average lifetime (0.81 ns) when compared to samples stored in a nitrogen/dark environment (8.3 ns). A photoetching technique commonly used for controlled reduction of QD size was found to induce energetic trap states to CdSe QDs and accelerate the rate of electron–hole recombination. X-ray absorption near edge structure (XANES) analysis confirms surface oxidation, the extent of which is shown to be dependent on the thickness of the ligand shell.
427. Electron Hopping Through Single-to-Few Layer Graphene Oxide Films. Photocatalytically Activated Metal Nanoparticle Deposition. Lightcap, I. V.; Murphy, S.; Schumer, T.; Kamat, P. V. J. Phys. Chem. Lett. 2012, 3, 1453–1458.
 Single- to few-layer graphene oxide (GO) sheets have been successfully anchored onto TiO2 films using electrophoretic deposition. Upon UV illumination of TiO2–GO films, photogenerated electrons from TiO2 are captured by GO. These electrons are initially used in GO's reduction, while additional electron transfer results in storage across its sp2 network. In the presence of silver ions, deposition of silver nanoparticles (NPs) is accomplished on the GO surface opposite the TiO2, thus confirming the ability of GO to transport electrons through its plane. Illumination-controlled reduction of silver ions allows for simple selection of particle size and loading, making these semiconductor–graphene–metal (SGM) films ideal for custom catalysis and sensor applications. Initial testing of SGM films as surface-enhanced resonance Raman (SERRS) sensors produced significant target molecule signal enhancements, enabling detection of nanomolar concentrations.
Single- to few-layer graphene oxide (GO) sheets have been successfully anchored onto TiO2 films using electrophoretic deposition. Upon UV illumination of TiO2–GO films, photogenerated electrons from TiO2 are captured by GO. These electrons are initially used in GO's reduction, while additional electron transfer results in storage across its sp2 network. In the presence of silver ions, deposition of silver nanoparticles (NPs) is accomplished on the GO surface opposite the TiO2, thus confirming the ability of GO to transport electrons through its plane. Illumination-controlled reduction of silver ions allows for simple selection of particle size and loading, making these semiconductor–graphene–metal (SGM) films ideal for custom catalysis and sensor applications. Initial testing of SGM films as surface-enhanced resonance Raman (SERRS) sensors produced significant target molecule signal enhancements, enabling detection of nanomolar concentrations.
426. Origin of Reduced Graphene Oxide Enhancements in Electrochemical Energy Storage. Radich, J. G.; Kamat, P. V. ACS Catal. 2012, 2, 807–816.
 Reduced graphene oxide (RGO) has become a common substrate upon which active intercalation materials are anchored for electrochemical applications such as supercapacitors and lithium ion batteries. The unique attributes of RGO, including high conductivity and porous macrostructure, are often credited for enhanced cycling and capacity performance. Here we focus on probing the electrochemical response of α-MnO2/RGO composite used as an electrode in a lithium ion battery cell and elucidating the mechanistic aspects of the RGO on the commonly observed improvements in cycling and capacity. We find that electron storage properties of RGO enables better electrode kinetics, more rapid diffusion of Li+ to intercalation sites, and a greater capacitance effect during discharge. Further investigation of the length of the one-dimensional nanowire morphology of the α-MnO2 has allowed us to differentiate between the innate characteristics of the MnO2 and those of the RGO. RGO coupled with long nanowires (>5 μm) exhibited the best performance in all tests and retained 150 mAh/g capacity after 20 cycles at 0.4C rate.
Reduced graphene oxide (RGO) has become a common substrate upon which active intercalation materials are anchored for electrochemical applications such as supercapacitors and lithium ion batteries. The unique attributes of RGO, including high conductivity and porous macrostructure, are often credited for enhanced cycling and capacity performance. Here we focus on probing the electrochemical response of α-MnO2/RGO composite used as an electrode in a lithium ion battery cell and elucidating the mechanistic aspects of the RGO on the commonly observed improvements in cycling and capacity. We find that electron storage properties of RGO enables better electrode kinetics, more rapid diffusion of Li+ to intercalation sites, and a greater capacitance effect during discharge. Further investigation of the length of the one-dimensional nanowire morphology of the α-MnO2 has allowed us to differentiate between the innate characteristics of the MnO2 and those of the RGO. RGO coupled with long nanowires (>5 μm) exhibited the best performance in all tests and retained 150 mAh/g capacity after 20 cycles at 0.4C rate.
425. Fortification of CdSe Quantum Dots with Graphene Oxide. Excited State Interactions and Light Energy Conversion. Lightcap, I. V.; Kamat, P. V. J. Am. Chem. Soc., 2012, 134 (16), 7109–7116.
 Graphene based 2-D carbon nanostructures provide new opportunities to fortify semiconductor based light harvesting assemblies. Electron and energy transfer rates from photoexcited CdSe colloidal quantum dots (QDs) to graphene oxide (GO) and reduced graphene oxide (RGO) were isolated by analysis of excited state deactivation lifetimes as a function of degree of oxidation and charging in (R)GO. Apparent rate constants for energy and electron transfer determined for CdSe–GO composites were 5.5 × 108 and 6.7 × 108 s–1, respectively. Additionally, incorporation of GO in colloidal CdSe QD films deposited on conducting glass electrodes was found to enhance the charge separation and electron conduction through the QD film, thus allowing three-dimensional sensitization. Photoanodes assembled from CdSe–graphene composites in quantum dot sensitized solar cells display improved photocurrent response (150%) over those prepared without GO.
Graphene based 2-D carbon nanostructures provide new opportunities to fortify semiconductor based light harvesting assemblies. Electron and energy transfer rates from photoexcited CdSe colloidal quantum dots (QDs) to graphene oxide (GO) and reduced graphene oxide (RGO) were isolated by analysis of excited state deactivation lifetimes as a function of degree of oxidation and charging in (R)GO. Apparent rate constants for energy and electron transfer determined for CdSe–GO composites were 5.5 × 108 and 6.7 × 108 s–1, respectively. Additionally, incorporation of GO in colloidal CdSe QD films deposited on conducting glass electrodes was found to enhance the charge separation and electron conduction through the QD film, thus allowing three-dimensional sensitization. Photoanodes assembled from CdSe–graphene composites in quantum dot sensitized solar cells display improved photocurrent response (150%) over those prepared without GO.
424. Synchronized energy and electron transfer processes in covalently linked CdSe-squaraine dye-TiO2 light harvesting assembly. Choi, H.; Santra, P. K.; Kamat, P. V. ACS Nano 2012, 6 (6), 5718–5726.
 Manipulation of energy and electron transfer processes in a light harvesting assembly is an important criterion to mimic natural photosynthesis. We have now succeeded in sequentially assembling CdSe quantum dot (QD) and squaraine dye (SQSH) on TiO2 film and couple energy and electron transfer processes to generate photocurrent in a hybrid solar cell. When attached separately, both CdSe QDs and SQSH inject electrons into TiO2 under visible–near-IR irradiation. However, CdSe QD if linked to TiO2 with SQSH linker participates in an energy transfer process. The hybrid solar cells prepared with squaraine dye as a linker between CdSe QD and TiO2 exhibited power conversion efficiency of 3.65% and good stability during illumination with global AM 1.5 solar condition. Transient absorption spectroscopy measurements provided further insight into the energy transfer between excited CdSe QD and SQSH (rate constant of 6.7 × 1010 s–1) and interfacial electron transfer between excited SQSH and TiO2 (rate constant of 1.2 × 1011 s–1). The synergy of covalently linked semiconductor quantum dots and near-IR absorbing squaraine dye provides new opportunities to harvest photons from selective regions of the solar spectrum in an efficient manner.
Manipulation of energy and electron transfer processes in a light harvesting assembly is an important criterion to mimic natural photosynthesis. We have now succeeded in sequentially assembling CdSe quantum dot (QD) and squaraine dye (SQSH) on TiO2 film and couple energy and electron transfer processes to generate photocurrent in a hybrid solar cell. When attached separately, both CdSe QDs and SQSH inject electrons into TiO2 under visible–near-IR irradiation. However, CdSe QD if linked to TiO2 with SQSH linker participates in an energy transfer process. The hybrid solar cells prepared with squaraine dye as a linker between CdSe QD and TiO2 exhibited power conversion efficiency of 3.65% and good stability during illumination with global AM 1.5 solar condition. Transient absorption spectroscopy measurements provided further insight into the energy transfer between excited CdSe QD and SQSH (rate constant of 6.7 × 1010 s–1) and interfacial electron transfer between excited SQSH and TiO2 (rate constant of 1.2 × 1011 s–1). The synergy of covalently linked semiconductor quantum dots and near-IR absorbing squaraine dye provides new opportunities to harvest photons from selective regions of the solar spectrum in an efficient manner.
423. Boosting the Efficiency of Quantum Dot Sensitized Solar Cells Through Modulation of Interfacial Charge Transfer. Kamat, P. V. Acc. Chem. Res. 2012, 45 (11), 1906–1915.
 The demand for clean energy will require the design of nanostructure-based light-harvesting assemblies for the conversion of solar energy into chemical energy (solar fuels) and electrical energy (solar cells). Semiconductor nanocrystals serve as the building blocks for designing next generation solar cells, and metal chalcogenides (e.g., CdS, CdSe, PbS, and PbSe) are particularly useful for harnessing size-dependent optical and electronic properties in these nanostructures.
The demand for clean energy will require the design of nanostructure-based light-harvesting assemblies for the conversion of solar energy into chemical energy (solar fuels) and electrical energy (solar cells). Semiconductor nanocrystals serve as the building blocks for designing next generation solar cells, and metal chalcogenides (e.g., CdS, CdSe, PbS, and PbSe) are particularly useful for harnessing size-dependent optical and electronic properties in these nanostructures.
This Account focuses on photoinduced electron transfer processes in quantum dot sensitized solar cells (QDSCs) and discusses strategies to overcome the limitations of various interfacial electron transfer processes. The heterojunction of two semiconductor nanocrystals with matched band energies (e.g., TiO2 and CdSe) facilitates charge separation. The rate at which these separated charge carriers are driven toward opposing electrodes is a major factor that dictates the overall photocurrent generation efficiency. The hole transfer at the semiconductor remains a major bottleneck in QDSCs. For example, the rate constant for hole transfer is 2–3 orders of magnitude lower than the electron injection from excited CdSe into oxide (e.g., TiO2) semiconductor. Disparity between the electron and hole scavenging rate leads to further accumulation of holes within the CdSe QD and increases the rate of electron–hole recombination. To overcome the losses due to charge recombination processes at the interface, researchers need to accelerate electron and hole transport.
The power conversion efficiency for liquid junction and solid state quantum dot solar cells, which is in the range of 5–6%, represents a significant advance toward effective utilization of nanomaterials for solar cells. The design of new semiconductor architectures could address many of the issues related to modulation of various charge transfer steps. With the resolution of those problems, the efficiencies of QDSCs could approach those of dye sensitized solar cells (DSSC) and organic photovoltaics.
422. Manipulation of Charge Transfer Across Semiconductor Interface. A Criterion that Cannot be Ignored in Photocatalyst Design. Kamat, P. V. J.Phys. Chem. Lett. 2012, 3 (5), 663–672. (Perspective article)
 The Perspective focuses on photoinduced electron transfer between semiconductor–metal and semiconductor–semiconductor nanostructures and factors that influence the rate of electron transfer at the interface. The storage and discharge properties of metal nanoparticles play an important role in dictating the photocatalytic performance of semiconductor–metal composite assemblies. Both electron and hole transfer across the interface with comparable rates are important in maintaining high photocatalytic efficiency and stability of the semiconductor assemblies. Coupled semiconductors of well-matched band energies are convenient to improve charge separation. Furthermore, semiconductor and metal nanoparticles assembled on reduced graphene oxide sheets offer new ways to design multifunctional catalyst mat. The fundamental understanding of charge-transfer processes is important in the future design of light-harvesting assemblies.
The Perspective focuses on photoinduced electron transfer between semiconductor–metal and semiconductor–semiconductor nanostructures and factors that influence the rate of electron transfer at the interface. The storage and discharge properties of metal nanoparticles play an important role in dictating the photocatalytic performance of semiconductor–metal composite assemblies. Both electron and hole transfer across the interface with comparable rates are important in maintaining high photocatalytic efficiency and stability of the semiconductor assemblies. Coupled semiconductors of well-matched band energies are convenient to improve charge separation. Furthermore, semiconductor and metal nanoparticles assembled on reduced graphene oxide sheets offer new ways to design multifunctional catalyst mat. The fundamental understanding of charge-transfer processes is important in the future design of light-harvesting assemblies.
421. Mn-Doped Quantum Dot Sensitized Solar Cells. A Strategy to Boost Efficiency over 5% Santra, P. K.; Kamat, P. V. J. Am. Chem. Soc. 2012, 134 (5), 2508–2511.
 To make Quantum Dot Sensitized Solar Cells (QDSC) competitive, it is necessary to achieve power conversion efficiencies comparable to other emerging solar cell technologies. By employing Mn2+ doping of CdS, we have now succeeded in significantly improving QDSC performance. QDSC constructed with Mn-doped-CdS/CdSe deposited on mesoscopic TiO2 film as photoanode, Cu2S/Graphene Oxide composite electrode, and sulfide/polysulfide electrolyte deliver power conversion efficiency of 5.4%.
To make Quantum Dot Sensitized Solar Cells (QDSC) competitive, it is necessary to achieve power conversion efficiencies comparable to other emerging solar cell technologies. By employing Mn2+ doping of CdS, we have now succeeded in significantly improving QDSC performance. QDSC constructed with Mn-doped-CdS/CdSe deposited on mesoscopic TiO2 film as photoanode, Cu2S/Graphene Oxide composite electrode, and sulfide/polysulfide electrolyte deliver power conversion efficiency of 5.4%.
420. Sun-believable Solar Paint. A Transformative One-Step Approach for Designing Nanocrystalline Solar Cells Genovese, M.; Lightcap, I. V.; Kamat, P. V. ACS Nano 2012, 6 (1), 865–872.
 A transformative approach is required to meet the demand of economically viable solar cell technology. By making use of recent advances in semiconductor nanocrystal research, we have now developed a one-coat solar paint for designing quantum dot solar cells. A binder-free paste consisting of CdS, CdSe, and TiO2 semiconductor nanoparticles was prepared and applied to conducting glass surface and annealed at 473 K. The photoconversion behavior of these semiconductor film electrodes was evaluated in a photoelectrochemical cell consisting of graphene–Cu2S counter electrode and sulfide/polysulfide redox couple. Open-circuit voltage as high as 600 mV and short circuit current of 3.1 mA/cm2 were obtained with CdS/TiO2–CdSe/TiO2 electrodes. A power conversion efficiency exceeding 1% has been obtained for solar cells constructed using the simple conventional paint brush approach under ambient conditions. Whereas further improvements are necessary to develop strategies for large area, all solid state devices, this initial effort to prepare solar paint offers the advantages of simple design and economically viable next generation solar cells.
A transformative approach is required to meet the demand of economically viable solar cell technology. By making use of recent advances in semiconductor nanocrystal research, we have now developed a one-coat solar paint for designing quantum dot solar cells. A binder-free paste consisting of CdS, CdSe, and TiO2 semiconductor nanoparticles was prepared and applied to conducting glass surface and annealed at 473 K. The photoconversion behavior of these semiconductor film electrodes was evaluated in a photoelectrochemical cell consisting of graphene–Cu2S counter electrode and sulfide/polysulfide redox couple. Open-circuit voltage as high as 600 mV and short circuit current of 3.1 mA/cm2 were obtained with CdS/TiO2–CdSe/TiO2 electrodes. A power conversion efficiency exceeding 1% has been obtained for solar cells constructed using the simple conventional paint brush approach under ambient conditions. Whereas further improvements are necessary to develop strategies for large area, all solid state devices, this initial effort to prepare solar paint offers the advantages of simple design and economically viable next generation solar cells.
2011

419. Supersensitization of CdS Quantum Dots with NIR Organic Dye: Towards the Design of Panchromatic Hybrid-Sensitized Solar Cells Choi, H.; Nicolaescu, R.; Paek, S.; Ko, J.; Kamat, P. V. ACS Nano 2011, 5, 9238–9245.
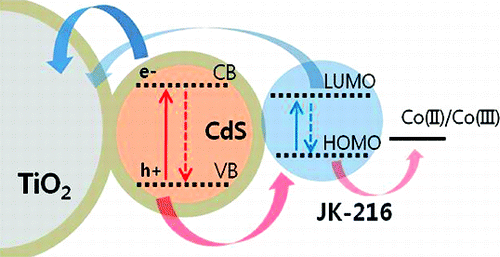 The photoresponse of quantum dot solar cells (QDSCs) has been successfully extended to the near-IR (NIR) region by sensitizing nanostructured TiO2–CdS films with a squaraine dye (JK-216). CdS nanoparticles anchored on mesoscopic TiO2 films obtained by successive ionic layer adsorption and reaction (SILAR) exhibit limited absorption below 500 nm with a net power conversion efficiency of 1% when employed as a photoanode in QDSC. By depositing a thin barrier layer of Al2O3, the TiO2–CdS films were further modified with a NIR absorbing squaraine dye. Quantum dot sensitized solar cells supersensitized with a squariand dye (JK-216) showed good stability during illumination with standard global AM 1.5 solar conditions, delivering a maximum overall power conversion efficiency (η) of 3.14%. Transient absorption and pulse radiolysis measurements provide further insight into the excited state interactions of squaraine dye with SiO2, TiO2, and TiO2/CdS/Al2O3 films and interfacial electron transfer processes. The synergy of combining semiconductor quantum dots and NIR absorbing dye provides new opportunities to harvest photons from different regions of the solar spectrum.
The photoresponse of quantum dot solar cells (QDSCs) has been successfully extended to the near-IR (NIR) region by sensitizing nanostructured TiO2–CdS films with a squaraine dye (JK-216). CdS nanoparticles anchored on mesoscopic TiO2 films obtained by successive ionic layer adsorption and reaction (SILAR) exhibit limited absorption below 500 nm with a net power conversion efficiency of 1% when employed as a photoanode in QDSC. By depositing a thin barrier layer of Al2O3, the TiO2–CdS films were further modified with a NIR absorbing squaraine dye. Quantum dot sensitized solar cells supersensitized with a squariand dye (JK-216) showed good stability during illumination with standard global AM 1.5 solar conditions, delivering a maximum overall power conversion efficiency (η) of 3.14%. Transient absorption and pulse radiolysis measurements provide further insight into the excited state interactions of squaraine dye with SiO2, TiO2, and TiO2/CdS/Al2O3 films and interfacial electron transfer processes. The synergy of combining semiconductor quantum dots and NIR absorbing dye provides new opportunities to harvest photons from different regions of the solar spectrum.
418. Cu2S-Reduced Graphene Oxide Composite for High Efficiency Quantum Dot Solar Cells . Overcoming the Redox Limitations of S2-/Sn2- at the Counter Electrode Radich, J. G.; Dwyer, R.; Kamat, P. V. J. Phys. Chem. Lett. 2011, 2, 2453–2460.
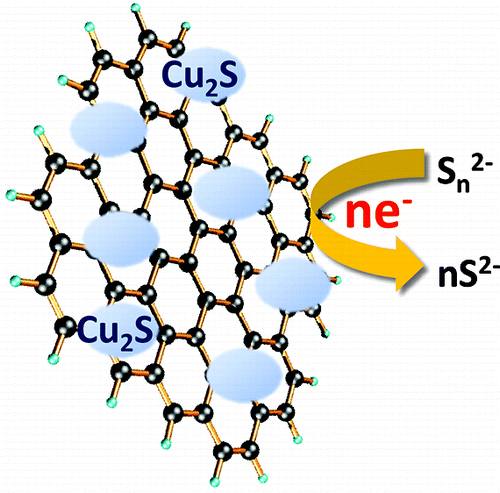 Polysulfide electrolyte that is employed as a redox electrolyte in quantum dot sensitized solar cells provides stability to the cadmium chalcogenide photoanode but introduces significant redox limitations at the counter electrode through undesirable surface reactions. By designing reduced graphene oxide (RGO)-Cu2S composite, we have now succeeded in shuttling electrons through the RGO sheets and polysulfide-active Cu2S more efficiently than Pt electrode, improving the fill factor by 75%. The composite material characterized and optimized at different compositions indicates a Cu/RGO mass ratio of 4 provides the best electrochemical performance. A sandwich CdSe quantum dot sensitized solar cell constructed using the optimized RGO-Cu2S composite counter electrode exhibited an unsurpassed power conversion efficiency of 4.4%.
Polysulfide electrolyte that is employed as a redox electrolyte in quantum dot sensitized solar cells provides stability to the cadmium chalcogenide photoanode but introduces significant redox limitations at the counter electrode through undesirable surface reactions. By designing reduced graphene oxide (RGO)-Cu2S composite, we have now succeeded in shuttling electrons through the RGO sheets and polysulfide-active Cu2S more efficiently than Pt electrode, improving the fill factor by 75%. The composite material characterized and optimized at different compositions indicates a Cu/RGO mass ratio of 4 provides the best electrochemical performance. A sandwich CdSe quantum dot sensitized solar cell constructed using the optimized RGO-Cu2S composite counter electrode exhibited an unsurpassed power conversion efficiency of 4.4%.
417. Role of Water Oxidation Catalyst, IrO2 in Shuttling Photogenerated Holes Across TiO2 Interface Meekins, B. H.; Kamat, P. V., J. Phys. Chem. Lett. 2011, 2, 2304-2310.
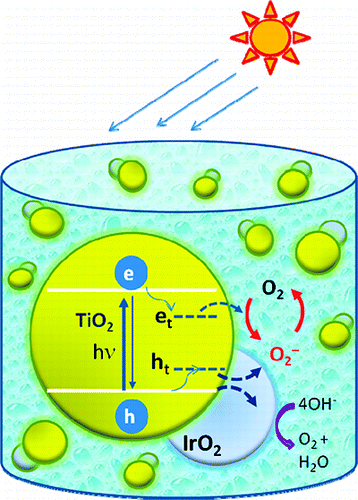 Iridium oxide, a water oxidation cocatalyst, plays an important role in mediating the hole transfer process of a UV-irradiated TiO2 system. Spectroscopic identification of trapped holes has enabled their characterization in colloidal TiO2 suspension and monitoring of the transfer of trapped holes to IrO2. Titration of trapped holes with potassium iodide yields an estimate of three holes per particle during 7 min of UV irradiation of TiO2 suspension in ethanol containing 5% acetic acid. The hole transfer to IrO2 occurs with a rate constant of 6 × 105 s–1. Interestingly, IrO2 also catalyzes the recombination of trapped holes with reduced oxygen species. The results discussed here provide a mechanistic and kinetic insight into the catalytic role of IrO2 in the photogenerated hole transfer process.
Iridium oxide, a water oxidation cocatalyst, plays an important role in mediating the hole transfer process of a UV-irradiated TiO2 system. Spectroscopic identification of trapped holes has enabled their characterization in colloidal TiO2 suspension and monitoring of the transfer of trapped holes to IrO2. Titration of trapped holes with potassium iodide yields an estimate of three holes per particle during 7 min of UV irradiation of TiO2 suspension in ethanol containing 5% acetic acid. The hole transfer to IrO2 occurs with a rate constant of 6 × 105 s–1. Interestingly, IrO2 also catalyzes the recombination of trapped holes with reduced oxygen species. The results discussed here provide a mechanistic and kinetic insight into the catalytic role of IrO2 in the photogenerated hole transfer process.
416. Capture, Store and Discharge. Shuttling Photogenerated Electrons across TiO2-Silver Interface Takai, A.; Kamat, P. V., ACS Nano 2011, 5, 7369–7376.
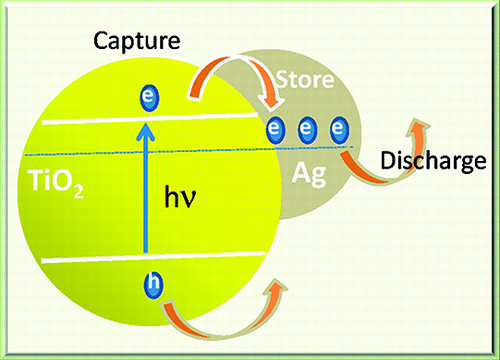 UV irradiation of TiO2 nanoparticles in the presence of Ag+ ions results in the quantitative reduction and deposition of silver on its surface. Continued UV irradiation following the deposition of Ag on the TiO2 surface causes a blue shift in the surface plasmon peak from 430 to 415 nm as these particles become charged with excess electrons. Under UV irradiation, both the charging and discharging of electrons occur at different rates, thus allowing the system to attain a steady state. Upon stopping the UV irradiation, a fraction of these electrons remain stored. The electron storage is dependent on the amount of Ag deposited on TiO2 nanoparticles with maximum capacity seen at 8.6 μM of Ag in a suspension containing 5.8 mM of TiO2. Such electron charging and discharging processes in semiconductor–metal composites need to be taken into account while evaluating the plasmon resonance induced effects in photocatalysis and photoelectrochemistry.
UV irradiation of TiO2 nanoparticles in the presence of Ag+ ions results in the quantitative reduction and deposition of silver on its surface. Continued UV irradiation following the deposition of Ag on the TiO2 surface causes a blue shift in the surface plasmon peak from 430 to 415 nm as these particles become charged with excess electrons. Under UV irradiation, both the charging and discharging of electrons occur at different rates, thus allowing the system to attain a steady state. Upon stopping the UV irradiation, a fraction of these electrons remain stored. The electron storage is dependent on the amount of Ag deposited on TiO2 nanoparticles with maximum capacity seen at 8.6 μM of Ag in a suspension containing 5.8 mM of TiO2. Such electron charging and discharging processes in semiconductor–metal composites need to be taken into account while evaluating the plasmon resonance induced effects in photocatalysis and photoelectrochemistry.
415. Charge-Transfer Complexation and Excited State Interactions in Porphyrin-Silver Nanoparticle Hybrid Nanostructures Murphy, S.; Huang, L.; Kamat, P. V. J. Phys. Chem. C 2011, 115 (46), pp 22761–22769.
 Highly photoactive porphyrin is shown to form charge-transfer complex with silver nanoparticles. Complexation of tetra(4-aminophenyl) porphyrin (TAPP) with Ag nanoparticles is confirmed by ground-state absorption and Raman spectroscopy. Strong Raman enhancement indicates both electromagnetic and chemical enhancement. Evidence of chemical enhancement includes a selective enhancement of porphyrin Raman bands. Fast charge separation in the complex is indicated by ultrafast transient absorption and fluorescence upconversion measurements. The charge-separated state is shown to have a lifetime of 116 ± 6 ps. Porphyrin substituents are shown to play a role in the formation of charge-transfer complex.
Highly photoactive porphyrin is shown to form charge-transfer complex with silver nanoparticles. Complexation of tetra(4-aminophenyl) porphyrin (TAPP) with Ag nanoparticles is confirmed by ground-state absorption and Raman spectroscopy. Strong Raman enhancement indicates both electromagnetic and chemical enhancement. Evidence of chemical enhancement includes a selective enhancement of porphyrin Raman bands. Fast charge separation in the complex is indicated by ultrafast transient absorption and fluorescence upconversion measurements. The charge-separated state is shown to have a lifetime of 116 ± 6 ps. Porphyrin substituents are shown to play a role in the formation of charge-transfer complex.
414. Electron Transfer Cascade by Organic/Inorganic Ternary Composites of Porphyrin, Zinc Oxide Nanoparticles, and Reduced Graphene Oxide on a Tin Oxide Electrode that Exhibits Efficient Photocurrent Generation Hayashi, H.; Lightcap, I. V.; Tsujimoto, M.; Takano, M.; Umeyama, T.; Kamat, P. V.; Imahori, H. J. Am. Chem. Soc. 2011, 133. 7684–7687.
 A bottom-up strategy has been developed to construct a multiple electron transfer system composed of organic/inorganic ternary composites (porphyrin, zinc oxide nanoparticles, reduced graphene oxide) on a semiconducting electrode without impairing the respective donor–acceptor components. The hierarchical electron transfer cascade system exhibited remarkably high photocurrent generation with an incident-photon-to-current efficiency of up to ca. 70%.
A bottom-up strategy has been developed to construct a multiple electron transfer system composed of organic/inorganic ternary composites (porphyrin, zinc oxide nanoparticles, reduced graphene oxide) on a semiconducting electrode without impairing the respective donor–acceptor components. The hierarchical electron transfer cascade system exhibited remarkably high photocurrent generation with an incident-photon-to-current efficiency of up to ca. 70%.
413. CdSe Quantum Dot-Fullerene Hybrid Nanocomposite for Solar Energy Conversion: Electron Transfer and Photoelectrochemistry Bang, J. H.; Kamat, P. V. ACS Nano 2011, 5 (12), pp 9421–9427.
 The development of organic/inorganic hybrid nanocomposite systems that enable efficient solar energy conversion has been important for applications in solar cell research. Nanostructured carbon-based systems, in particular C60, offer attractive strategies to collect and transport electrons generated in a light harvesting assembly. We have assembled CdSe–C60 nanocomposites by chemically linking CdSe quantum dots (QDs) with thiol-functionalized C60. The photoinduced charge separation and collection of electrons in CdSe QD–C60 nanocomposites have been evaluated using transient absorption spectroscopy and photoelectrochemical measurements. The rate constant for electron transfer between excited CdSe QD and C60 increased with the decreasing size of the CdSe QD (7.9 × 109 s–1 (4.5 nm), 1.7 × 1010 s–1 (3.2 nm), and 9.0 × 1010 s–1 (2.6 nm)). Slower hole transfer and faster charge recombination and transport events were found to dominate over the forward electron injection process, thus limiting the deliverance of maximum power in CdSe QD–C60-based solar cells. The photoinduced charge separation between CdSe QDs and C60 opens up new design strategies for developing light harvesting assemblies.
The development of organic/inorganic hybrid nanocomposite systems that enable efficient solar energy conversion has been important for applications in solar cell research. Nanostructured carbon-based systems, in particular C60, offer attractive strategies to collect and transport electrons generated in a light harvesting assembly. We have assembled CdSe–C60 nanocomposites by chemically linking CdSe quantum dots (QDs) with thiol-functionalized C60. The photoinduced charge separation and collection of electrons in CdSe QD–C60 nanocomposites have been evaluated using transient absorption spectroscopy and photoelectrochemical measurements. The rate constant for electron transfer between excited CdSe QD and C60 increased with the decreasing size of the CdSe QD (7.9 × 109 s–1 (4.5 nm), 1.7 × 1010 s–1 (3.2 nm), and 9.0 × 1010 s–1 (2.6 nm)). Slower hole transfer and faster charge recombination and transport events were found to dominate over the forward electron injection process, thus limiting the deliverance of maximum power in CdSe QD–C60-based solar cells. The photoinduced charge separation between CdSe QDs and C60 opens up new design strategies for developing light harvesting assemblies.
412. Quantum Dot Solar Cells Tvrdy, K.; Kamat, P. V., In Comprehensive Nanoscience and Technology, D. L. Andrews; Scholes, G. D. and Wiederrecht, G. P., Editors: Oxford Academic Press, 2011; p.257-275 (Book Chapter)
411. Understanding the Role of the Sulfide Redox Couple (S2-/Sn2-) in Quantum Dot Sensitized Solar Cells Chakrapani, V.; Baker, D.; Kamat, P. V. J. Am. Chem. Soc. 2011, 133, 9607-9615.
 The presence of sulfide/polysulfide redox couple is crucial in achieving stability of metal chalcogenide (e.g., CdS and CdSe)-based quantum dot-sensitized solar cells (QDSC). However, the interfacial charge transfer processes play a pivotal role in dictating the net photoconversion efficiency. We present here kinetics of hole transfer, characterization of the intermediates involved in the hole oxidation of sulfide ion, and the back electron transfer between sulfide radical and electrons injected into TiO2 nanoparticles. The kinetic rate constant (107–109 s–1) for the hole transfer obtained from the emission lifetime measurements suggests slow hole scavenging from CdSe by S2– is one of the limiting factors in attaining high overall efficiency. The presence of the oxidized couple, by addition of S or Se to the electrolyte, increases the photocurrent, but it also enhances the rate of back electron transfer.
The presence of sulfide/polysulfide redox couple is crucial in achieving stability of metal chalcogenide (e.g., CdS and CdSe)-based quantum dot-sensitized solar cells (QDSC). However, the interfacial charge transfer processes play a pivotal role in dictating the net photoconversion efficiency. We present here kinetics of hole transfer, characterization of the intermediates involved in the hole oxidation of sulfide ion, and the back electron transfer between sulfide radical and electrons injected into TiO2 nanoparticles. The kinetic rate constant (107–109 s–1) for the hole transfer obtained from the emission lifetime measurements suggests slow hole scavenging from CdSe by S2– is one of the limiting factors in attaining high overall efficiency. The presence of the oxidized couple, by addition of S or Se to the electrolyte, increases the photocurrent, but it also enhances the rate of back electron transfer.
410. Tracking the Adsorption and Electron Injection Rates of CdSe Quantum Dots on TiO2: Linked Versus Direct Attachment Pernik, D.; Tvrdy, K.; Radich, J. G.; Kamat, P. V. J. Phys. Chem. C 2011, 133 (24), pp 9607–9615.
 Understanding CdSe quantum dot (QD) adsorption phenomena on mesoscopic TiO2 films is important for improving the performance of quantum dot sensitized solar cells (QDSSCs). A kinetic adsorption model has been developed to elucidate both Langmuir-like submonolayer adsorption and QD aggregation processes. Removal of surface-bound trioctylphosphine oxide as well as the use of 3-mercaptopropionic acid (MPA) as a molecular linker improved the adsorption of toluene-suspended QDs onto TiO2 films. The adsorption constant Kad for submonolayer coverage was (6.7 ± 2.7) × 103 M–1 for direct adsorption and (4.2 ± 2.0) × 104 M–1 for MPA-linked assemblies. Prolonged exposure of a TiO2 film to a CdSe QD suspension resulted in the assembly of aggregated particles regardless of the method of adsorption. A greater coverage of TiO2 was achieved with smaller QDs due to reduced size constraints. Ultrafast transient absorption spectroscopy demonstrated faster electron injection into TiO2 from directly adsorbed QDs (kET = 7.2 × 109 s–1) compared with MPA-linked QDs (kET = 2.3 × 109 s–1). The adsorption kinetic details presented in this study are useful for controlling CdSe QD adsorption on TiO2 and designing efficient photoanodes for QDSSCs.
Understanding CdSe quantum dot (QD) adsorption phenomena on mesoscopic TiO2 films is important for improving the performance of quantum dot sensitized solar cells (QDSSCs). A kinetic adsorption model has been developed to elucidate both Langmuir-like submonolayer adsorption and QD aggregation processes. Removal of surface-bound trioctylphosphine oxide as well as the use of 3-mercaptopropionic acid (MPA) as a molecular linker improved the adsorption of toluene-suspended QDs onto TiO2 films. The adsorption constant Kad for submonolayer coverage was (6.7 ± 2.7) × 103 M–1 for direct adsorption and (4.2 ± 2.0) × 104 M–1 for MPA-linked assemblies. Prolonged exposure of a TiO2 film to a CdSe QD suspension resulted in the assembly of aggregated particles regardless of the method of adsorption. A greater coverage of TiO2 was achieved with smaller QDs due to reduced size constraints. Ultrafast transient absorption spectroscopy demonstrated faster electron injection into TiO2 from directly adsorbed QDs (kET = 7.2 × 109 s–1) compared with MPA-linked QDs (kET = 2.3 × 109 s–1). The adsorption kinetic details presented in this study are useful for controlling CdSe QD adsorption on TiO2 and designing efficient photoanodes for QDSSCs.
409. Graphene-based Composites for Electrochemical Energy Storage Radich, J. G.; McGinn, P. J.; Kamat, P. V. Interface 2011, Spring Issue, 63-66.
.png) This article focuses on graphene-based electrodes for
electrochemical energy conversion and storage devices.
As elaborated in the other feature articles in this issue,
graphene is a 2D “flat mat” consisting of a honeycomb-like
structure of carbon atoms with sp2 bonding character for
each carbon. It exhibits excellent electrical conductivity and
mechanical strength, and can be synthesized in a number
of ways. Most syntheses involve first oxidizing graphite to
graphene oxide (GO), a widely used method developed many
years ago and referred to as Hummers Method. The graphitic
carbon is dissolved in sulfuric acid along with other oxidants
such as sodium nitrate during sonication, and KMnO4 is
slowly added to complete the oxidation process. Washing
and rinsing allows for a fully exfoliated GO suspension in
water, ethanol, and other polar solvents, that can be prepared
as a stable colloid for months. The GO can be subsequently
reduced in a number of ways by UV irradiation or thermal
treatment, sonolysis, or chemical treatment with a strong
reducing agent such as hydrazine and is referred to as
reduced graphene oxide (RGO). The use of RGO in lithium ion
batteries is still in its infancy and may provide a practical and
inexpensive way to substantially improve the performance of
these electrochemical energy storage devices, serving markets
ranging from the long-sought electric vehicle as well as simple
applications such as cell phones and laptop computers.
GO is an extraordinarily useful material for the development
of composite materials as a result of the oxygen moieties
spread throughout the formerly-pristine carbon sheet. These
highly electronegative species allow for the stability of the
GO colloidal suspension as well as the binding of cations.
Binding cationic or organic protonated anions (i.e., acetate)
produces GO composites, and subsequently RGO composites
that are formed from further chemical processing. The ability
to selectively bind cationic materials has led to a swarm of
activity surrounding the development of novel GO/RGOnanoparticle composite materials for a variety of applications.
The development of electrodes for lithium ion batteries using
the following scheme has produced a number of RGO-metal
oxide composites already.
This article focuses on graphene-based electrodes for
electrochemical energy conversion and storage devices.
As elaborated in the other feature articles in this issue,
graphene is a 2D “flat mat” consisting of a honeycomb-like
structure of carbon atoms with sp2 bonding character for
each carbon. It exhibits excellent electrical conductivity and
mechanical strength, and can be synthesized in a number
of ways. Most syntheses involve first oxidizing graphite to
graphene oxide (GO), a widely used method developed many
years ago and referred to as Hummers Method. The graphitic
carbon is dissolved in sulfuric acid along with other oxidants
such as sodium nitrate during sonication, and KMnO4 is
slowly added to complete the oxidation process. Washing
and rinsing allows for a fully exfoliated GO suspension in
water, ethanol, and other polar solvents, that can be prepared
as a stable colloid for months. The GO can be subsequently
reduced in a number of ways by UV irradiation or thermal
treatment, sonolysis, or chemical treatment with a strong
reducing agent such as hydrazine and is referred to as
reduced graphene oxide (RGO). The use of RGO in lithium ion
batteries is still in its infancy and may provide a practical and
inexpensive way to substantially improve the performance of
these electrochemical energy storage devices, serving markets
ranging from the long-sought electric vehicle as well as simple
applications such as cell phones and laptop computers.
GO is an extraordinarily useful material for the development
of composite materials as a result of the oxygen moieties
spread throughout the formerly-pristine carbon sheet. These
highly electronegative species allow for the stability of the
GO colloidal suspension as well as the binding of cations.
Binding cationic or organic protonated anions (i.e., acetate)
produces GO composites, and subsequently RGO composites
that are formed from further chemical processing. The ability
to selectively bind cationic materials has led to a swarm of
activity surrounding the development of novel GO/RGOnanoparticle composite materials for a variety of applications.
The development of electrodes for lithium ion batteries using
the following scheme has produced a number of RGO-metal
oxide composites already.
408. Electron Transfer between Methyl Viologen Radicals and Graphene Oxide: Reduction, Electron Storage and Discharge Krishnamurthy, S.; Lightcap, I. V.; Kamat, P. V. J. Photochem. Photobiol. A:Chem. 2011, 221, 214-219.
 Photochemically generated methyl viologen radicals undergo electron transfer with graphene oxide (GO) in ethanol suspensions. This charge transfer interaction results in the reduction of GO as well as storage of electrons. The stored electrons can be utilized to reduce Ag+ ions and thus anchor silver nanoparticles on reduced graphene oxide (RGO). The spectroscopic experiments that elucidate the quantitative electron transfer and transmission electron microscopy that highlights the potential of designing metal–RGO assemblies are discussed.
Photochemically generated methyl viologen radicals undergo electron transfer with graphene oxide (GO) in ethanol suspensions. This charge transfer interaction results in the reduction of GO as well as storage of electrons. The stored electrons can be utilized to reduce Ag+ ions and thus anchor silver nanoparticles on reduced graphene oxide (RGO). The spectroscopic experiments that elucidate the quantitative electron transfer and transmission electron microscopy that highlights the potential of designing metal–RGO assemblies are discussed.
407. Virtual Issue: Graphene and Functionalized Graphene Prezhdo, O. V.; Kamat, P. V.; Schatz, G. C. J. Phys. Chem. C 2011, 115 (8), 3195-3197.
406. Graphene-based Nanoassemblies for Energy Conversion Kamat, P. V. J. Phys. Chem. Lett. 2011, 2, 242-251. (Perspective article)
405. Photoinduced electron transfer from semiconductor quantum dots to metal oxide nanoparticles Tvrdy, K.; Frantszov, P.; Kamat, P. V. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 29-34.
 Quantum dot-metal oxide junctions are an integral part of next-generation solar cells, light emitting diodes, and nanostructured electronic arrays. Here we present a comprehensive examination of electron transfer at these junctions, using a series of CdSe quantum dot donors (sizes 2.8, 3.3, 4.0, and 4.2 nm in diameter) and metal oxide nanoparticle acceptors (SnO2, TiO2, and ZnO). Apparent electron transfer rate constants showed strong dependence on change in system free energy, exhibiting a sharp rise at small driving forces followed by a modest rise further away from the characteristic reorganization energy. The observed trend mimics the predicted behavior of electron transfer from a single quantum state to a continuum of electron accepting states, such as those present in the conduction band of a metal oxide nanoparticle. In contrast with dye-sensitized metal oxide electron transfer studies, our systems did not exhibit unthermalized hot-electron injection due to relatively large ratios of electron cooling rate to electron transfer rate. To investigate the implications of these findings in photovoltaic cells, quantum dot-metal oxide working electrodes were constructed in an identical fashion to the films used for the electron transfer portion of the study. Interestingly, the films which exhibited the fastest electron transfer rates (SnO2) were not the same as those which showed the highest photocurrent (TiO2). These findings suggest that, in addition to electron transfer at the quantum dot-metal oxide interface, other electron transfer reactions play key roles in the determination of overall device efficiency.
Quantum dot-metal oxide junctions are an integral part of next-generation solar cells, light emitting diodes, and nanostructured electronic arrays. Here we present a comprehensive examination of electron transfer at these junctions, using a series of CdSe quantum dot donors (sizes 2.8, 3.3, 4.0, and 4.2 nm in diameter) and metal oxide nanoparticle acceptors (SnO2, TiO2, and ZnO). Apparent electron transfer rate constants showed strong dependence on change in system free energy, exhibiting a sharp rise at small driving forces followed by a modest rise further away from the characteristic reorganization energy. The observed trend mimics the predicted behavior of electron transfer from a single quantum state to a continuum of electron accepting states, such as those present in the conduction band of a metal oxide nanoparticle. In contrast with dye-sensitized metal oxide electron transfer studies, our systems did not exhibit unthermalized hot-electron injection due to relatively large ratios of electron cooling rate to electron transfer rate. To investigate the implications of these findings in photovoltaic cells, quantum dot-metal oxide working electrodes were constructed in an identical fashion to the films used for the electron transfer portion of the study. Interestingly, the films which exhibited the fastest electron transfer rates (SnO2) were not the same as those which showed the highest photocurrent (TiO2). These findings suggest that, in addition to electron transfer at the quantum dot-metal oxide interface, other electron transfer reactions play key roles in the determination of overall device efficiency.
2010

404. Photocatalytic Events of CdSe Quantum Dots in Confined Media. Electrodic Behavior of Coupled Platinum Nanoparticles Harris, C.; Kamat, P. V. ACS Nano 2010, 4 (12), 7312-7330.
403. Reduced Graphene Oxide and Porphyrin. An Interactive Affair in 2-D Wojcik, A.; Kamat, P. V. ACS Nano 2010, 4, 6697–6706.
402. Capturing Hot Electrons Kamat, P. V. Nature Chemistry 2010, 2, 809-810. (News & Views)
401. Beyond photovoltaics: semiconductor nanoarchitectures for liquid junction solar cells Kamat, P. V.; Tvrdy, K.; Baker, D. R.; Radich, J. G. Chem. Rev. 2010, 110, 6664–6688.
400. To What Extent Do Graphene Scaffolds Improve the Photovoltaic and Photocatalytic Response of TiO2 Nanostructured Films? Ng, Yun Hau, Lightcap, I. V.; Goodwin, K.; Matsumura, M.; Kamat, P. V. J. Phys. Chem. Lett. 2010 1, 2222-2227.
399. Sonolytic Design of Graphene Au Nanocomposites. Simultaneous and Sequential Reduction of Graphene Oxide and Au(III) Vinodgopal, K. Neppolian, B.; Lightcap, I. V.; Grieser, F.; Ashokkumar, M.; Kamat, P. V. J. Phys. Chem. Lett. 2010 1, 1987-1993.
398. Revealing Surface Interactions in Quantum Dot Based Photovoltaic Architectures (A Perspective on the Article, Electronic Energy Alignment at the PbSe Quantum Dots/ZnO(1010) Interface by Timp and Zhu) Kamat, P. V. Surf. Sci. 2010 604, 1331-1332.
397. Tuning the Emission of CdSe Quantum Dots by Controlled Trap Enhancement Baker, D. R.; Kamat, P. V., Langmuir 2010,26, 11272–11276. NDRL 484
396. Photochemistry of Far Red Responsive Tetrahydroquinoxaline-Based Squaraine Dyes Wojcik, A.; Nicolaescu, R.; Kamat, P. V.; Patil, S. J. Phys. Chem. A 2010, 114, 2744-2750. NDRL 4841
395. Solar Cell by Design. Photoelectrochemistry of TiO2 Nanorod Arrays Decorated with CdSe Bang, J. H.; Kamat, P. V. Adv. Funct. Mater. 2010, 20, 1970-1976. NDRL 4839
394. Graphene-Based Nanoarchitectures. Anchoring Semiconductor and Metal Nanoparticles on a 2-Dimensional Carbon Support Kamat, P. V. J. Phys. Chem. Lett. 2010, 1, 520-527. (Perspective)
393. Modulation of Electron Injection in CdSe-TiO2 System through Medium Alkalinity Chakrapani, V.; Tvrdy, K.; Kamat, P. V. J. Am. Chem. Soc. 2010, 132, 1228-1229.
392. CdSe Nanowire Photoelectrochemical Solar Cells Enhanced with Colloidal CdSe Quantum Dots Yu, Y.; Kamat, P. V.; Kuno, M. Adv. Funct. Mater. 2010, in press. NDRL 48.
391. Anchoring Semiconductor and Metal Nanoparticles on a 2-Dimensional Catalyst Mat. Storing and Shuttling Electrons with Reduced Graphene Oxide Lightcap, I. V.; Kosel, T. H.; Kamat, P. V. Nano Lett. 2010, 4, 577–583.
390. Supramolecular Donor-Acceptor Assemblies Composed of Carbon Nanodiamond and Porphyrin for Photoinduced Electron Transfer and Photocurrent Generation Ohtani, M.; Kamat, P. V.; Fukuzumi, S. J. Mater. Chem. 2010, 20, 582-587.
389. Squaraine Rotaxane as Optical Chloride Sensor Gassensmith, J. J.; Matthys, S.; Wojcik, A.; Kamat, P. V.; Smith, B. D. Chemistry, Euro. J. 2010, 16, 2916-2921. NDRL 4817
388. Tailored TiO2–SrTiO3 Heterostructure Nanotube Arrays for Improved Photoelectrochemical Performance Zhang, J.; Bang, J. H.; Tang, C.; Kamat, P. V. ACS Nano 2010, 4, 387-395. NDRL 4812
2009
387. Photosensitization of SnO2 and Other Dyes Kamat, P. V. In Dye Sensitized Solar Cells; K. Kalyansundaram, Ed.; 2009, EPFL Press: Laussane, Switzerland. NDRL 4818 (Book Chapter)
386. Nanotechnology for Next Generation Solar Cells Kamat, P. V.; Schatz, G. J. Phys. Chem. C 2009, 15473–15475. (Editorial)
385. Got TiO2 Nanotubes? Lithium Ion Intercalation can Boost Their Photoelectrochemical Performance Three-Fold Meekins, B. H.; Kamat, P. V. ACS Nano 2009, 3, 3437–3446. NDRL 4821
384. Fuel Cell Geared in Reverse. Photocatalytic Hydrogen Production using a TiO2/Nafion/Pt Membrane Assembly with No Applied Bias Seger, B.; Kamat, P. V. J. Phys. Chem. C 2009, 113,18946–18952. NDRL 4820
383. Disassembly, Reassembly and Photoelectrochemistry of Etched TiO2 Nanotubes Baker, D. R.; Kamat, P. V. J. Phys. Chem. C 2009, 113, 17967-17972. NDRL 4816
382. CdSe Quantum Dot Sensitized Solar Cells. Shuttling Electrons through Stacked Carbon Nanocups Farrow, B.; Kamat, P. V. J. Am. Chem. Soc 2009, 131, 11124-11131. NDRL 4804
381. Quantum Dot Sensitized Solar Cells. A Tale of Two Semiconductor Nanocrystals: CdSe and CdTe Bang, J. H.; Kamat, P. V. ACS Nano 2009, 3, 1467-1476. NDRL 4800
380. Graphene-Semiconductor Nanocomposites. Excited State Interactions between ZnO Nanoparticles and Graphene Oxide Williams, G.; Kamat, P. V. Langmuir 2009, 25, 13869-13873. NDRL 4798
379. Photocatalysis with CdSe Nanoparticles in Confined Media: Mapping Charge Transfer Events in the Subpicosecond to Second Timescales Harris, C. T.; Kamat, P. V. ACS Nano 2009, 3, 682-690. NDRL 4790
377. Substrate driven photochemistry of CdSe Quantum Dot Films: Charge Injection and Irreversible Transformation on Oxide Surfaces Tvrdy, K.; Kamat, P. J. Phys. Chem. C 2009, 113, 3765-3772. NDRL 4780
376. Photodegradation of Polythiophene Based Polymers. Excited State Properties and Radical Intermediates Koch, M.; Nicolaescu, R.; Kamat, P. V. J. Phys. Chem. B 2009, 113, 11507-11513. NDRL 4778
375. Photosensitization of TiO2 Nanostructures with CdS Quantum Dots. Particulate versus Tubular Support Architectures Baker, D. R.; Kamat, P. V. Adv. Funct. Mater. 2009, 19, 805-811. NDRL 4771
2008

374. Quantum Dot Solar Cells. Semiconductor Nanocrystals as Light Harvestors - Centennial Feature Article Kamat, P. V. J. Phys. Chem. C 2008, 112, 18737-18753 . NDRL 4770
373. Excited State and Photoelectrochemical Behavior of Pyrene Linked Phenyleneethynylene Oligomer Matsunaga, Y.; Takechi, K.; Akasaka, T.; Ramesh, A. R.; James, P. V.; Thomas, K. G.;Kamat, P. V. J. Phys. Chem. B 2008, 112, 14539-14547. NDRL 4768
372. Layer-by-layer self-assembly of colloidal gold-silica multilayers Zhang, Z.; Meisel, D.; Kamat, P.;Kuno, M. Chem. Educator 2008, 13, 153-157. NDRL 4736
371. TiO2-Graphene Nanocomposites. UV-Assisted Photocatalytic Reduction of Graphene Oxide Williams, G.; Seger, B.; Kamat, P. V. ACS Nano 2008, 2.1487-1491 NDRL 4763
370. Quantum Dot Solar Cells. Electrophoretic Deposition of CdSe-C60 Composite Films and Capture of Photogenerated Electrons with nC60 Cluster Shell Brown, P.; Kamat, P. V. J. Am. Chem. Soc. 2008, 130 8890–8891. NDRL 4762
369. Decorating Graphene Sheets with Gold Nanoparticles Muszynski, R.; Seger, B.; Kamat, P. J. Phys. Chem. C 2008, 112, 5263 - 5266. NDRL 4760
368. Single-Walled Carbon Nanotube Scaffolds for Dye-Sensitized Solar Cells Brown, P. R.; Takechi, K.; Kamat, P. V. J. Phys. Chem. C 2008, 112 4776-4782. NDRL 4754
367. Quantum Dot Solar Cells. Tuning Photoresponse through Size and Shape Control of CdSe-TiO2 Architecture Kongkanand, A.; Tvrdy, K.; Takechi, K.; Kuno, M. K.; Kamat, P. V. J. Am. Chem. Soc. 2008, 130 4007 - 4015. NDRL 4752
366. Solvent Dependence of the Charge-Transfer Properties of a Quaterthiophene-Anthraquinone Dyad Wan, J.; Ferreira, A.; Xia, W.; Chow, C. H.; Takechi, K.; Kamat, P. V.; Guilford Jones, I.; Vullev, V. I. J. Photochem. A 2008, 197, 364-374. NDRL 4744
365. Harvesting Infrared Photons with Croconate Dyes Takechii, K Kamat, P. V., Avirah, R. R., Jyothish. K.; Ramaiah, D. Chem. Mater. 2008, 20, 265-272. NDRL 4737
364. Fullerene-Based Supramolecular Nanoclusters with poly[2-methoxy-5-(2'-ethylhexyloxy)-p-phenylenevinylene] (MEH-PPV) for Light Energy Conversion Hasobe, T.; Fukuzumi, S.; Kamat, P.V.; Murata, H. Jap. J. Appl. Phys. 2008, 47, 1223-1229. NDRL 4734
363. Platinum Dispersed on Silica Nanoparticles for PEM Fuel Cells Seger, B.; Kongkanand, A.; Vinodgopal, K.; Kamat, P.V. J. Electroanal. Chem. 2008, 621, 198-204. NDRL 4730.
2007

362. Photoelectrochemistry of stacked cup carbon nanotube films. Tube-Length dependence and charge transfer with excited porphyrin Hasobe, T.; Kamat, P.V. J. Phys. Chem. C 2007, 111, 16626-16634. NDRL 4733
361. Hydroxyl Radical Mediated Degradation of Phenylarsonic Acid Xu, T., Kamat, P. V., Joshi, S., Mebel, Yong Cai, A. M. and O'Shea, K. J. Phys. Chem. B 2007, 111, 7819-7824. NDRL 4738
360. Electron Storage in Single Wall Carbon Nanotubes. Fermi Level Equilibration in Semiconductor–SWCNT Suspensions Kongkanand, A.; Kamat, P.V. ACS Nano 2007, 1, 13-21. NDRL 4722
359. Interactions of Single Wall Carbon Nanotubes with Methyl Viologen Radicals. Quantitative Estimation of Stored Electrons Kongkanand, A.; Kamat, P.V. J. Phys. Chem. C 2007, 111, 9012-9015. NDRL 4722
358. Ru(II)trisbipyridine Functionalized Gold Nanorods. Morphological Changes and Excited-State Interactions Jebb, M.; Sudeep, P.K.; Pramod, P.; Thomas, K.G.; Kamat, P.V. J. Phys. Chem. B 2007, 111, 6839 - 6844. NDRL 4709
357. Size-Dependent electron Injection from Excited CdSe Quantum Dots into TiO2 Nanoparticles Robel, I.; Kuno, M.; Kamat, P. V. J. Am. Chem. Soc. 2007, 129 (14), 4136 -4137. NDRL 4707

356. Porphyrin based molecular architectures for light energy conversion Hasobe, T.; Fukuzumi, S.; Kamat, P.V.; Murata, H. Mol. Cryst. Liq. Cryst. 2007, 471, 39-51. NDRL 4706
355. Proton activity in Nafion films. Probing exchangeable protons with methylene blue. Seger, B.; Vinodgopal, K.; Kamat, P. V. Langmuir 2007, 23, 5471-5476. NDRL 4704
354. Anchoring ZnO Particles on Functionalized Single Wall Carbon Nanotubes. Excited State Interactions and Charge Collection. Vietmeyer, F.; Seger, B.; Kamat, P. V. Adv. Mater. 2007, 19, 2935-2940. NDRL 4701
353. Meeting the Clean Energy Demand: Nanostructure Architectures for Solar Energy Conversion. Kamat, P. V. J. Phys. Chem. C 2007, 111, 2834-2860. (Feature Article in February 22 2007 issue) NDRL 4697
352. Shape- and Functionality-Controlled Organization of TiO2-Porphyrin-C60 Assembly for Improved Performance of Photochemical Solar Cells Chemistry. Hasobe, T.; Fukuzumi, S.; Hattori, S.; Kamat, P. V. Asian J. 2007, 2, 265-272.
351. Single Wall Carbon Nanotube Scaffolds for Photoelectrochemical Solar Cells. Capture and Transport of Photogenerated Electrons. Kongkanand, A.; Domínguez, R. M.; Kamat, P. V. Nano Lett. 2007, 7, 676-680. NDRL 4698
350. Singlet Oxygen Generation Using Iodinated Squaraine and Squaraine-Rotaxane Dyes. Arunkumar, E.; Sudeep, P. K.; Kamat, P. V.; Noll, B. C.; Smith, B. D. New J. Chem. 2007, 31, 677-683. NDRL 4700
349. Organic Solar Cells. Supramolecular Composites of Porphyrins and Fullerenes Organized by Polypeptide Structures as Light Harvesters. Hasobe, T.; Saito, K.; Kamat, P.V.; Troiani, V.; Qiu, H.; Solladié, N.; Kim, K.S.; Park, J.K.; Kim, D.; D'Souza, F.; Fukuzumi, S., J. Mater. Chem. 2007, 17 4160-4170. NDRL 4680
348. Harvesting Photons in the Infrared. Electron Injection from Excited Tricarbocyanine dye (IR 125) into TiO2 and Ag@TiO2 core-shell nanoparticles. Sudeep, P.K.; Takechi, K.; Kamat, P.V., J. Phys. Chem. C 2007, 111, 488-494. NDRL 4678
2006

347. Harvesting Photons with Carbon Nanotubes. Kamat, P. V. Nanotoday 2006, 1, 20-27. NDRL 4646
346. Photochemistry of Ruthenium Trisbipyridine Functionalized on Gold Nanoparticles. Pramod, P.; Sudeep, P.K.; Thomas, K.G.; Kamat, P.V. J. Phys. Chem. B 2006, 110, 20737-20747.
345. Organized Assemblies of Single-Wall Carbon Nanotube (SWCNT) and Porphyrin for Photochemical Solar Cells. Charge Injection from Excited Porphyrin into SWCNT. Hasobe, T.; Fukuzumi, S.; Kamat, P.V., J. Phys. Chem. B 2006, 110, 16169-16173. NDRL 4879
344. Hierarchial Assembly of Porphyrins and Fullerenes for Solar Cells. Hasobe, T.; Fukuzumi, S.; Kamat, P.V. Interface 2006, 15, 47-51. NDRL 4660
343. Highly-dispersed Pt catalysts on Single-Walled Carbon Nanotubes and Their Role in Methanol Oxidation. Kongkanand, A.; Vinodgopal, K.; Kuwabata, S.; Kamat, P.V. J. Phys. Chem. B 2006, 110, 16185-16192. NDRL 4673
342. Harvesting Infrared Photons with Tricarbocyanine Dye Clusters. Takechi, K.; Sudeep, S.; Kamat, P.V. J. Phys. Chem. B 2006, 110, 16169-16173. NDRL 4669
341. Probing photochemical transformations at TiO2/Pt and TiO2/Ir interfaces using x-ray absorption spectroscopy. Lahiri, D.; Subramanian, V.; Bunker, B.A.; Kamat, P.V. J. Chem. Phys. 2006, 124, 204720. NDRL 4662
340. Terahertz All-Optical Molecule-Plasmon Modulation. Dintinger, J.; Robel, I.; Kamat, P.V.; Genet, C.; Ebbesen, T.W. Advanced Materials 2006, 18, 1645-1648. NDRL 4657
339. Exciton Recombination in CdSe nanowires. Bimolecular to three-particle Auger Kinetics. Robel, I.; Bunker, B. A.; Kamat, P. V.; Kuno, M. K. Nano Lett. 2006, 6, 1344-1349. NDRL 4647

338. Carbon Nanomaterials: Building Blocks in Energy Conversion Devices. Kamat, P.V. Interface 2006, 15, 45-47 NDRL 4646
337. Singlet and Triplet Excited State Interactions and Photochemical Reactivity of Phenyleneethynylene Oligomers. Sudeep, P.K.; James, P.V.; Thomas, K.G.; Kamat, P.V. J. Phys. Chem. A 2006, 110, 5642-5649. NDRL 4645
336. Extending the Photoresponse of TiO2 to the Visible Light Region: Photoelectrochemical Behavior of TiO2 Thin Films Prepared by RF-Magnetron Sputtering Deposition Method. Kikuchi, H.; Kitano, M.; Takeuchi, M.; Matsuoka, M.; Anpo, M.; Kamat, P.V. J. Phys. Chem. B 2006, 110, 5537 - 5541. NDRL 4644
335. Quantum Dot Solar Cells. Harvesting Light Energy with CdSe Nanocrystals Molecularly Linked to Mesoscopic TiO2 Films. Robel, I., Subramanian, V., Kuno, M. and Kamat, P. V. J. Am. Chem. Soc. 2006, 128 (7), 2385-2393. NDRL 4627
334. Single Wall Carbon Nanotube Supports for Portable Direct Methanol Fuel Cells. Girishkumar, G., Hall, T. D., Vinodgopal, K. and Kamat, P. V. J. Phys. Chem. B 2006, 110, 107-114. NDRL 4625
333. Stacked-Cup Carbon Nanotubes for Photoelectrochemical Solar Cells. Hasobe, T.; Fukuzumi, S.; Kamat, P.V. Angew. Chem. (Int. Ed.) 2006, 45, 755-759. NDRL 4623
332. Structural changes and catalytic activity of platinum nanoparticles supported on C60 and carbon nanotube films during the operation of direct methanol fuel cells. Robel, I.; Girishkumar, G.; Bunker, B.A.; Kamat, P.V.; Vinodgopal, K. Appl. Phys. Lett. 2006,88, 073113. NDRL 4619
331. Supramolecular nanostructured assemblies of different types of porphyrins with fullerenes using TiO2 nanoparticles for light energy conversion. Hasobe, T.; Hattori, S.; Kamat, P.V.; Fukuzumi, S. Tetrahedron 2006, 62, 1937-1946. NDRL 4606
2005
330. Single-Wall Carbon Nanotubes Supported Platinum Nanoparticles with Improved Electrocatalytic Activity of Oxygen Reduction. Kongkanand, A., Kuwabata, S., Girishkumar, G. and Kamat, P. Langmuir 2005, 21, 2392-2396. NDRL 4631
329. Mechanistic Evaluation of Arsenite Oxidation in TiO2 Assisted photocatalysis. Xua, T., Kamat, P. V. and O'Shea, K. E. J. Phys. Chem. A 2005, 109, 9070-9075. NDRL 4624
328. Photosensitized Growth of Silver Nanoparticles under Visible Light Irradiation: A Mechanistic Investigation. Sudeep, P. K. and Kamat, P. V. Chem. Mater. 2005, 17, 5404-5410. NDRL 4611
327. Radiolytic Transformations of Chlorinated Phenols and Chlorinated Phenoxyacetic Acids. Peller, J. and Kamat, P. V. J. Phys. Chem. A 2005, 105, 9528-9535. NDRL 4609
326. Organization of supramolecular assemblies of fullerene porphyrin and fluorescein dye derivatives on TiO2 nanoparticles for light energy conversion. Hasobe, T., Hattori, S., Kamat, P. V., Urano, Y., Umezawa, N., Nagano, T. and Fukuzumi, S. Chem. Phys. 2005, 319, 243-252. NDRL 4608
325. SWCNT-CdS nanocomposite as light harvesting assembly. Photoinduced charge transfer interactions. Robel, I., Bunker, B. and Kamat, P. V. Adv. Mater. 2005, 17, 2458-2463. NDRL 4583
324. Ordered assembly of protonated porphyrin driven by SWCNT- J and H aggregates to Nanorods. Hasobe, T., Fukuzumi, S. and Kamat, P. V. J. Am. Chem. Soc. 2005, 127 (34) 11884-11885. NDRL 4587
323. Photoinduced Electron Transfer Processes in Fullerene-Based Donor-Acceptor Systems. Thomas, K. G., George, M. V. and Kamat, P. V. Helv. Chim. Acta 2005, 88, 1291-1308. NDRL 4584

322. Boosting the Fuel Cell Performance with a Semiconductor Photocatalyst. TiO2/Pt-Ru Hybrid Catalyst for Methanol Oxidation. Drew, K., Girishkumar, G., Vinodgopal, K. and Kamat, P. V. J. Phys. Chem. B 2005, 109, 11851 - 11857. NDRL 4583
321. The spectroelectrochemistry of aromatic amine oxidation. An insight into the indo dye formation. Pillai, Z. S. and Kamat, P. V. Res. Chem. Intermed. 2005, 31, 103-112. NDRL 4526
320. A Drastic Difference in Lifetimes of the Charge-Separated State of Formanilide-Anthraquinone Dyad vs Ferrocene-Formanilide- Anthraquinone Triad and Their Photoelectrochemical Properties of the Composite Films with Fullerene Clusters. Okamoto, K., Hasobe, T., Tkachenko, N. V., Lemmetyinen, H., Prashant V. Kamat and Fukuzumi, S. J. Phys. Chem. A 2005, 109, 4662-4670. NDRL 4578
319. Single Wall Carbon Nanotube based Proton Exchange Membrane Assembly for Hydrogen Fuel Cells. Girishkumar, G., Rettker, M., Underhile, R., Binz, D., Vinodgopal, K., McGinn, P. and Kamat, P. Langmuir 2005, 21, 8487 - 8494. NDRL 4575
318. Charge Separation and Catalytic Activity of Ag@TiO2 Core-Shell Composite Clusters under UV-Irradiation. Hirakawa, T. and Kamat, P. V. J. Am. Chem. Soc. 2005, 3928-3934. NDRL 4574
317. Mechanistic pathways of the hydroxyl radical reactions of quinoline. 1. Identification, distribution and yields of hydroxylated products. Nicolaescu, A. R., Wiest, O. and Kamat, P. V. J. Phys. Chem. A 2005, 109, 2822-2828. NDRL 4563
316. Mechanistic pathways of the hydroxyl radical reactions of quinoline. 2. Computational analysis of .OH attack at C-atoms. Nicolaescu, A. R., Wiest, O. and Kamat, P. V. J. Phys. Chem. A 2005, 109, 2829-2835. NDRL 4564
315. Organization of supramolecular assembly of 9-mesityl-10-carboxymethylacridinium ion and fullerene clusters on TiO2 nanoparticles for light energy conversion. Hasobe, T., Hattori, S., Kamat, P. V., Wada, Y. and Fukuzumi, S. J. Mater. Chem. 2005, 15, 372-380. NDRL 4560
314. Enhancement of Light-Energy Conversion Efficiency by Multi-Porphyrin Arrays of Porphyrin-Peptide Oligomers with Fullerene Clusters. Hasobe, T., Kamat, P. V., Troiani, V., Solladie, N., Ahn, T. K., Kim, S. K., Kim, D., Kongkanand, A., Kuwabata, S. and Fukuzumi, S. J. Phys. Chem. B 2005, 109, 19-23. NDRL 4542
313. Photoinduced Electron Transfer between Chlorophyll a and Gold Nanoparticles. Barazzouk, S., Kamat, P. V. and Hotchandani, S. J. Phys. Chem. B 2005, 109, 716-723. NDRL 4541
312. Photovoltaic Cells using composite nanoclusters of porphyrins and fullerenes with gold nanoparticles. Hasobe, T., Imahori, H., Kamat, P. V. and Fukuzumi, S. J. Am. Chem. Soc. 2005, 127, 1216-1228. NDRL 4525
2004

311. Hydroxyl Radical's Role in the Remediation of a Common Herbicide, 2,4-Dichlorophenoxyacetic acid (2,4-D). Peller, J., Wiest, O. and Kamat, P. V. J. Phys. Chem. A 2004, 108, 10925-10933 (Feature Article). NDRL 4544
310. Single wall carbon nanotube films for photocurrent generation. A prompt response to visible light irradiation. Barazzouk, S., Hotchandani, S., Vinodgopal, K. and Kamat, P. V. J. Phys. Chem. B 2004, 108, 17015-17018. NDRL 4540 (See supporting information for the electrodeposition movie)
309. Photoelectrochemical Properties of Supramolecular Composite of Fullerene Nanoclusters and 9-Mesityl-10-Carboxymethyl-acridiniium Ion on SnO2. Hasobe, T., Hattori, S., Kotani, H., Ohkubo, K., Hosomizu, K., Imahori, H., Kamat, P. V. and Fukuzumi, S. Org. Lett. 2004, 6, 3103-3106. NDRL 4518
308. Carbon Nanostructures in Portable Fuel Cells: Single-Walled Carbon Nanotube Electrodes for Methanol Oxidation and Oxygen Reduction. Girishkumar, G., Vinodgopal, K., Meisel, D. and Kamat, P. V. J. Phys. Chem. B 2004, 108, 19960 - 19966. NDRL 4538
307. Supramolecular Photovoltaic Cells Based on Composite Molecular Nanoclusters: Dendritic Porphyrin and C60, Porphyrin Dimer and C60, and Porphyrin-C60 Dyad. Hasobe, T., Kamat, P. V., Absalom, M. A., Kashiwagi, Y., Sly, J., Crossley, M. J., Hosomizu, K., Imahori, H. and Fukuzumi, S. J. Phys. Chem. B 2004, 108, 12865-12872. NDRL 4523
306. Self-Assembled Linear Bundles of Single Wall Carbon Nanotubes and Their Alignment and Deposition as a Film in a DC-Field. Kamat, P. V., Thomas, K. G., Barazzouk, S., Girishkumar, G., Vinodgopal, K. and Meisel, D. J. Am. Chem. Soc. 2004, 126, 10757-10762. Movies 1 &2 showing the real time alignment of carbon nanotubes
305. Electron Storage and Surface Plasmon Modulation in Ag@TiO2 Clusters. Hirakawa, T. and Kamat, P. V. Langmuir 2004, 20, 5645-5647. NDRL 4518
304. pi-Complex formation in electron-transfer reactions of porphyrins. Shunichi Fukuzumi, Taku Hasobea, Ohkuboa, K., Crossley, M. J., Kamat, P. V. and Imahori, H. J. Porphyrins and Phthalocyanines 2004, 8, (2), 191-200. NDRL 4514
303. C60 Cluster as an Electron Shuttle in a Ru(II)-Polypyridyl Sensitizer Based Photochemical Solar Cell. Kamat, P. V., Haria, M. and Hotchandani, S. J. Phys. Chem. B 2004, 108, 5166-5170. NDRL 4506
302. Catalysis with TiO2/Au Nanocomposites. Effect of Metal Particle Size on the Fermi Level Equilibration. Subramanian, V., E.E. Wolf, and P.V. Kamat J. Am. Chem. Soc. 2004, 126, 4943-4950. NDRL 4496
301. Uniaxial Plasmon Coupling through Longitudinal Self-Assembly of Gold Nanorods. George Thomas, K., S. Barazzouk, B.I. Ipe, S.T. Shibu Joseph, and P.V. Kamat J. Phys. Chem. B 2004, 108, 13066-13068. NDRL 4495

300. Fullerene Based Carbon Nanostructures for Methanol Oxidation. Vinodgopal, K., M. Haria, D. Meisel, and P. Kamat Nano Lett. 2004, 3, 415-418. NDRL 4492
299. Supramolecular Photovoltaic Cells Using Porphyrin Dendrimers and Fullerene. Hasobe, T., Y. Kashiwagi, M.A. Absalom, Kohei Hosomizu, M.J. Crossley, H. Imahori, P.V. Kamat, and S. Fukuzumi Adv. Mater. 2004, 16, 975-978. NDRL 4489
298. Semiconductor Nanostructures For Detection and Degradation Of Low Level Organic Contaminants From Water. In Nanotechnology and the Environment: Applications and Implications Kamat, P.V., R. Huhen, and R. Nicolaescu, P.Alivisatos, et al., Ed.; 2004, The American Chemical Society: Washington, D.C.
297. The Selective Electrochemical Detection of Model Pollutant Species Using Films of Naturally Occurring Humic Acid K. Environ. Vinodgopal, K., V. Subramanian, and P.V. Kamat Sci. Technol. 2004, 38, 2161-2166 NDRL 4471
296. Photochemistry and Electrochemistry of Nanoassemblies. Kamat, P.V., In Chemistry of Nanomaterials, C. N. R. Rao, A. Muller, and A.K. Cheetham, Editors. 2004, John Wiley Interscience: New York. Vol. 2, pp 620-645
2003
295. What Factors Control the Size and Shape of Silver Nanoparticles in the Citrate Ion Reduction Method. Pillai, Z.S. and P.V. Kamat J. Phys. Chem. B 2003, 107,945-951. NDRL 4482
294. C60/C60- Redox couple as a probe in the Determination of Fermi Level of Semiconductor Nanoparticles. Jacob, M., Levanon, H., Kamat, P. V. In Fullerenes and Nanotubes, Pamat, P. V., Guldi, D. M., D'Souza, F., Ed.; Electrochemical Society, 2003, p 9-12.
293. Quaternary Self-Organization of Porphyrin and Fullerene Units by Clusterization with Gold Nanoparticles on SnO2 Electrodes for Organic Solar Cells. Hasobe, T., H. Imahori, S. Fukuzumi, and P.V. Kamat J. Am. Chem. Soc. 2003, 125, 14962-14963 NDRL 4466
292. Light Energy Harvesting Using Mixed Molecular Nanoclusters. Porphyrin and C60 Cluster Films for Efficient Photocurrent Generation. Hasobe, T., H. Imahori, S. Fukuzumi, and P.V. Kamat J. Phys. Chem. B 2003, 107, 12105 - 12112. NDRL 4464
291. Nanostructured assembly of porphyrin clusters for light energy conversion. Hasobe, T., H. Imahori, S. Fukuzumi, and P.V. Kamat J. Mater. Chem. 2003, 13, 2515 - 2520. NDRL 4462
290. Photoinduced Electron Transfer Processes in Fullerene Based Dyads with Heteroaromatic Donors. George Thomas, K., V. Biju, D.M. Guldi, P.V. Kamat, and M.V. George Chem. Phys. Chem. 2003, 4, 1299-1307. NDRL 4147
289. Mass-Transfer and Kinetic Studies during the Photocatalytic Degradation of an Azo Dye on Optically Transparent Electrode Thin Film. Subramanian, V., P.V. Kamat, and E. Wolf Ind. Eng. Chem. Res. 2003, 42, 2131-2138. NDRL 4398
288. Nanoscience Opportunities in Environmental Remediation. Kamat, P.V. and D. Meisel Comptes Rendus Chimie 2003, 6, 999-1007. NDRL 4443 (Review Article)
287. Chromophore-Functionalized Gold Nanoparticles. K George Thomas, PV Kamat Acc. Chem. Res. 2003, 36 (12) 888-898. NDRL 4440 (Review Article)
286. Photoinduced charge transfer between CdSe nanocrystals and p-phenelenediamine. Sharma, S.; Pillai, Z.S.; Kamat, P.V. J. Phys. Chem. B 2003, 107,10088-10093. NDRL 4436
285. Charge Distribution between UV-Irradiated TiO2 and Gold Nanoparticles. Determination of Shift in Fermi Level. Jakob, M.; Levanon, H.; Kamat, P.V. Nano Lett. 2003, 3(3), 353-358. NDRL 4429
284. Green Emission to Probe Photoinduced Charging Events in ZnO-Au Nanoparticles. Charge Distribution and Fermi-Level Equilibration. Subramanian, V.; Wolf, E.E.; Kamat, P.V. J. Phys. Chem. B 2003, 107, 7479-7485. NDRL 4422
283. Radical induced oxidative transformations of Quinoline. Nicolaescu, R.; Wiest, O.; Kamat, P. V. J. Phys. Chem. A 2003, 107, 427-433. NDRL 4414
282. Synergy of combining sonolysis and photocatalysis in the degradation and mineralization of chlorinated aromatic compounds. Peller, J.; Wiest, O.; Kamat, P. V. Environ. Sci. Technol. 2003, 37, 1926-1932. NDRL 4409
281. Photoinduced transformations at semiconductor/metal interfaces: X-ray absorption studies of titania/gold films. Dey, D. L.; Subramanian, V.; T.Shibata; Wolf, E. E.; Bunker, B. A.; Kamat, P. V. J. Appl. Phys. 2003, 93 (5) 2575-2581. NDRL 4405
280. Molecular Assembly of Fullerenes as Nanoclusters and Nanostructured Films. Kamat, P. V.; George Thomas, K. In Nanoscale Materials, L. Liz-Marzan and Kamat, P. V., Ed.; 2003, Kluwer Academic/Plenum Publishers: Boston. p. 475-494. NDRL 4404
279. Electrophoretic Assembly of Naturally Occurring Humic Substances as Thin Films. Vinodgopal, K.; Subramanian, V.; Carrasquillo, S.; Kamat, P. V. Environ. Sci. Technol. 2003, 37 (4), 761-765. NDRL 4399
278. Charge Transfer on the Nanoscale: Current Status. Adams, D.; Brus, L.; Chidsey, C. E. D.; Creager, S.; Cruetz, C.; Kagan, C. R.; Kamat, P. V.; Lieberman, M.; Lindsay, S.; Marcus, R. A.; Metzger, R. M.; Michel-Beyerle, M. E.; Miller, J. R.; Newton, M. D.; Rolison, D. R.; Sankey, O.; Schanze, K. S.; Yardley, J.; Zhu, X. J. Phys. Chem. B 2003, 107, 6668-6697. NDRL 4397
277. Influence of Metal/Metal Ion Concentration on the Photocatalytic Activity of TiO2−Au Composite Nanoparticles. Subramanian, V.; Wolf, E. E.; Kamat, P. V. Langmuir 2003, 19, 469-474. NDRL 4384
276. Mechanism of Hydroxyl Radical-Induced Breakdown of the Herbicide 2,4-Dichlorophenoxyacetic Acid. Peller, J.; Wiest, O.; Kamat, P. V. Chemistry, European J. 2003, 9, 5379-5387. NDRL 4351
2002

275. Nanoparticles in Advanced Oxidation Processes. Kamat, P. V.; Meisel, D. Current Opinion in Colloid & Interface Science 2002 7 282-287. NDRL 4394
274. Molecular Assembly of Fullerenes as Nanoclusters and Films. Kamat, P. V.; Barazzouk, S.; George Thomas, K.; George, M. V., In Fullerenes-2002, P. V. Kamat, Guldi, D. M., and D'Souza, F., Ed.; 2002, The Electrochemical Society: Pennigton, NJ. NDRL 4389
273. Photophysical, photochemical and photocatalytic aspects of metal nanoparticles. Kamat, P. V. J. Phys. Chem. B 2002, 106, 7729-7744. NDRL 4374 (Feature Article)
272. Photoinduced Transformations in Semiconductor-Metal Nanocomposite Assemblies. Kamat, P. V. Pure Appl. Chem. 2002,74, 1693-1706. NDRL 4370
271. Tuning the Properties of CdSe Nanoparticles in Reverse Micelles. Chandrasekharan, N.; Kamat, P. V. Res. Chem. Intermed. 2002, 28, 847-856. NDRL 4367
270. Electrochemical Modulation of Fluorophore Emission at a Nanostructured Gold Film. Kamat, P. V.; Barazzouk, S.; Hotchandani, S. Angew. Chem. (Int. Ed.) 2002, 41, 2764-2767. NDRL 4354
269. Surface Binding Properties of Tetraoctylammomium Bromide Capped Gold nanoparticles. George Thomas, K.; Zajicek, J.; Kamat, P. V. Langmuir 2002, 18, 3722-3727. NDRL 4347
268. Unusual Electrocatalytic Behavior of Ferrocene Bound Fullerene Cluster Films. Barazzouk, S.; Hotchandani, S.; Kamat, P. V. J. Mater. Chem. 2002, 12, 2021-2025. NDRL 4346

267. Inter-Particle Electron Transfer between Size-Quantized CdS and TiO2 Semiconductor Nanoclusters. Sant, P. A.; Kamat, P. V. Phys. Chem. Chem. Phys. 2002, 4, 198-203. NDRL 4330
266. Clusters of Bis- and Tris-Fullerenes. Biju, V.; Sudeep, P. K.; George Thomas, K.; George, M. V.; Barazzouk, S.; Kamat, P. V. Langmuir 2002, 18, 1831-1839, NDRL 4328
265. Fullerene Functionalized Gold Nanoparticles. A self assembled Photoactive Antenna-Metal Nanocore Assembly. Sudeep, P. K.; Ipe, B. I.; George Thomas, K.; George, M. V.; Barazzouk, S.; Hotchandani, S.; Kamat, P. V. Nano Lett. 2002, 2, 29-35. NDRL 4322
264. Photoinduced Charge Separation in a Fluorophore-Gold Nanoassembly. Ipe, B. I.; George Thomas, K.; Barazzouk, S.; Hotchandani, S.; Kamat, P. V. J. Phys. Chem. B 2002, 106, 18-21. NDRL 4319
263. A Sense and Shoot Approach for Photocatalytic Degradation of Organic Contaminants in Water. Kamat, P. V.; Huehn, R.; Nicolaescu, R. J. Phys. Chem. B 2002, 106, 788-794. NDRL 4307
262. Metal-metal and metal-semiconductor composite nanoclusters. Kamat, P.V.; Flumiani, M.; Dawson, A., Colloids and Surfaces A. Physicochemical and Engineering Aspects 2002, 202, 269-279. NDRL 4275
2001
261. Electrochemical Aspects of C60-Ferrocene Cluster Films. Kamat, P. V.; Barazzouk, S.; Hotchandani, S. In Fullerenes-2001, P. V. Kamat, Guldi, D. M., and Kadish, K., Ed.; 2001, The Electrochemical Society: Pennigton, NJ. p 41-47 NDRL 4301
260. Spectroscopy and Photocurrent Generation in Nanostructured Thin Films of Porphyrin-Fullerene Dyad Clusters. Hiroshi Imahori, Taku Hasobe, Hiroko Yamada, Prashant V. Kamat, Said Barazzouk, Mamoru Fujitsuka, Osamu Ito, and Shunichi Fukuzumi Chem. Lett. 2001, 784-785, NDRL 4300
259. Nanostructured Fullerene Films. Kamat, P. V.; Barazzouk, S.; Hotchandani, S. Adv. Mater. 2001, 13, 1614-1617. NDRL 4290
258. Semiconductor-Metal Composite Nanostructures. To What Extent Metal Nanoparticles (Au, Pt, Ir) Improve the Photocatalytic Activity of TiO2 Films? Subramanian, V.; Wolf, E.; Kamat, P. V. J. Phys. Chem. B 2001, 105, 11439-11446. NDRL 4289
257. Electrochromic and Photoelectro-chromic Aspects of Semiconductor Nanostructure- Molecular Assembly. Kamat, P. V., In The Electrochemistry of Nanostructures, G. Hodes, Ed.; 2001, Wiley-VCH: New York. p. 229-246. NDRL 426
256. Photoinduced Electron Transfer Between 1,2,5-Triphenylpyrrolidinofullerene Cluster Aggregates and Electron Donors. Biju, V.; Barazzouk, S.; George Thomas, K.; George, M.V.; Kamat, P.V., Langmuir 2001, 17, 2930-2936. NDRL 4278
255. Radiation induced catalytic dechlorination of hexachlorobenzene on various oxide surfaces. Zacheis, G.A.; Gray, K.A.; Kamat, P.V., J. Phys. Chem. 2001, 105, 4715-4720 . NDRL 4259
254. Semiconductor-metal nanocomposites. Photoinduced fusion and photocatalysis of gold capped TiO2 (TiO2/Au) nanoparticles. Dawson, A. ; Kamat, P.V., J. Phys. Chem. B 2001, 105, 960-966. NDRL 4258
253. Sonolysis of 2,4-Dichlorophenoxyacetic Acid in Aqueous Solutions. Evidence for •OH-Radical-Mediated Degradation. Peller, J., Wiest, O. Kamat, P,V, J. Phys. Chem. A 2001, 105, 3176-3181. NDRL 4151
252. Assembling Gold Nanoparticles as Nanostructured Films Using an Electrophoretic Approach. Chandrasekharan, N. ; Kamat, P.V., Nano Lett. 2001, 67-70. NDRL 4228
2000
251. Metal Nanoparticles. How Noble Are They in the Light? Kamat, P.V., IAPS News Letter 2000, November, 34-42 NDRL 4276.
250. Understanding the Facile Photooxidation of Ru(bpy)32+ in Strongly Acidic Aqueous Solution Containing Dissolved Oxygen. Das, A., Joshi, V., Kotkar, D., Pathak, V.S., Swayambunathan, V., Kamat, P.V.Ghosh, P.K., J. Phys. Chem.A 2000, 105, 6945-6954. NDRL 4247
249. Complexation of Gold Nanoparticles with Radiolytically Generated Thiocyanate Radicals ((SCN)2-·). Dawson, A. Kamat, P.V., J. Phys. Chem. B 2000, 104, 11842-11846 . NDRL 4257
248. Dye Capped Gold Nanoclusters: Photoinduced changes in Gold/Rhodamine 6G Nanoassemblies. Chandrasekharan, N., Kamat, P.V., Hu, J.Jones II, G., J. Phys. Chem. B 2000, 104(47), 11103-11109. NDRL 4237
247. Nanostructured Thin Films of C60-Anline Dyad Clusters. Electrodeposition, Charge Separation and Photoelectrochemistry. Kamat, P.V., Barazzouk, S., Hotchandani, S.George Thomas, K., Chemistry, European J. 2000, 6(21), 3914-3921. NDRL 4219
246. Improving the Photoelectrochemical Performance of Nanostructured TiO2 Films by Adsorption of Gold Nanoparticles. Chandrasekharan, N. Kamat, P.V., J. Phys. Chem. B 2000, 104, 10851-10857. NDRL 4213
245. Combinative Sonolysis and Photocatalysis for Textile Dye Degradation. Stock, N.L., Peller, J., Vinodgopal, K.Kamat, P.V., Environ. Sci. & Technol. 2000, 34(9), 1747-1750. NDRL 4177
244. Making Gold Nanoparticles Glow. Enhanced emission from a Surface Bound Probe. George Thomas, K. Kamat, P.V., J. Am. Chem. Soc. 2000, 122(11), 2655 - 2656. NDRL 4172
243. Electrodeposition of C60 Clusters on Nanostructured SnO2 Films for Enhanced Photocurrent Generation. Kamat, P.V., Barazzouk, S., George Thomas, K.Hotchandani, S., J. Phys. Chem. B 2000, 104(17), 4814-1817. NDRL 4170
242. Excited Pinacyanol H-Aggregates and Their Interaction with SiO2 and SnO2 Nanoparticles. Barazzouk, S., Lee, H., Hotchandani, S.Kamat, P.V., J. Phys. Chem. B 2000, 104(15), 3616-3623. NDRL 4155
241. Radiolytic Reduction of Hexachlorobenzene in Surfactant Solutions: A Steady-State and Pulse Radiolysis Study. Zacheis, G.A., Gray, K.A.Kamat, P.V., Environ. Sci. Technol. 2000, 34(16), 3401-3407. NDRL 4152
240. Photophysical properties of pristine fullerenes, functionalized fullerenes and fullerenes containing donor-bridge-acceptor systems. Guldi, D.M. Kamat, P.V., In Fullerenes: Chemistry, Physics, and Technology, K. Kadish and R. Ruoff, Ed.; 2000, John Wiley & Sons, Inc.: New York. p. 225-282. NDRL 4125
239. A Simple Photocatalytic Experiment to Generate Fullerene Anions. Dawson, A; Kamat, P. V. In Fullerenes 2000: Chemistry and Physics of Fullerenes and Carbon Nanoclusters, Kamat, P. V., Guldi, D. M., Kadish, K. M., Ed.; The Electrochemical Society: Pennington, 2000 (PV2000-12); Vol.10; pp34-39.
1999
238. Redox characteristics of Schiff base manganese and cobalt complexes related to water-oxidizing complex of photosynthesis. Hotchandani, S., Ozdemir, U., Nasr, C., Allakhverdiev, S.I., Karacan, N., Klimov, V.V., Kamat, P.V., and Carpentier, R., Bioelectrochemistry and Bioenergetics 1999, 48(1), 53-59.
237. Orientation dependent electron transfer processes in Fullerene-Aniline Dyads. George Thomas, K., Biju, V., George, M.V., Guldi, D.M., and Kamat, P.V., J. Phys. Chem. B 1999, 103, 10755-10763. NDRL 4137
236. Onium Salt Effects on p-Terphenyl-Sensitized Photoreduction of Water to Hydrogen. Fujiwara, H., Kitamura, T., Wada, Y., Yanagida, S., and Kamat, P.V., J. Phys. Chem. A 1999, 103, 4874-4878. NDRL 4134
235. Photochemical Behavior of Anthraquinone Based Textile Dye (Uniblue-A) bound to Cellulose Powder and Cotton Fabric. Kamat, P.V., Das, S., Padmaja, S., and Madison, S.A., Res. Chem. Intermed. 1999, 25(9), 915-924. NDRL 4117
234. Controlling Dye (Merocyanine-540) Aggregation on nanostructured TiO2 Films. An Organized Assembly Approach for Enhancing the Efficiency of Photosensitization. Khazraji, A.C., Hotchandani, S., Das, S., and Kamat, P.V., J. Phys. Chem. B 1999, 103, 4693-4700. NDRL 4110
233. Free Radical Induced Oxidation of the Azo Dye Acid Yellow 9. Kinetics and Reaction Mechanism. Das, S., Kamat, P.V., Padmaja, S., Au, V., and Madison, S.A., J. Chem. Soc., Perkin Trans. 2 1999, (6), 1219 - 1224. NDRL 4105
232. Visible laser induced Fusion and fragmentation of thionicotinamide capped gold nanoparticles. Fujiwara, H., Yanagida, S., and Kamat, P.V., J. Phys. Chem. B 1999, 103, 2589-2591. NDRL 4095
231. Can H-Aggregates Serve as Light-Harvesting Antennae? Triplet−Triplet Energy Transfer between Excited Aggregates and Monomer Thionine in Aersol-OT Solutions. Das, S. and Kamat, P.V., J. Phys. Chem. B 1999, 103(1), 209-215. NDRL 4094
230. Photoinduced Charge Separation and Stabilization in Clusters of a Fullerene-Aniline Dyad. George Thomas, K., Biju, V., George, M.V., Guldi, D.M., and Kamat, P.V., J. Phys. Chem. B 1999, 103(42), 8864-8869. NDRL 4083
229. Radiation Chemical Processes on Oxide Surfaces. Catalytic degradation of Hexachlorobenzene on alumina nanoparticles. Zacheis, G.A., Gray, K.A., and Kamat, P.V., J. Phys. Chem. B 1999, 103, 2142-2150. NDRL 4076
228. Photoinduced charge separation in fullerene-aniline dyads. Biju, V., George Thomas, K., George, M.V., Guldi, D.M., and Kamat, P.V., In Recent Advances in the Chemistry and Physics of Fullerenes and Related materials. P.V. Kamat, D.M. Guldi, and K. Kadish, Ed.; 1999, The Electrochemical Society: Pennington, N. J. p. 296-303.
227. Excited state processes of fullerenes and functionalized fullerenes. An overview.
226. Semiconductor Nanoparticles. Kamat, P.V., Murakoshi, K., Wada, Y., and Yanagida, S., In Semiconductor Nanoparticles, H. Nalwa, Ed.; 1999, Academic Press: New York. p. 292-344. NDRL 4068
1998
225. Spectral Characterization of One Electron Oxidation Product of Ruthenium(II)cis-di(isothiocyanato)bis(4,4'-dicarboxy-2,2'-bipyridyl) Complex Using Pulse Radiolysis. Das, S.; Kamat, P. V., J. Phys. Chem. B 1998, 102, 8954-8957. NDRL 4082
224. Photoelectrochemistry of composite semiconductor thin films. II. Photosensitization of SnO2/TiO2 coupled system with a ruthenium polypyridyl complex. Nasr, C.; Hotchandani, S.; Kamat, P. V., J. Phys. Chem. B 1998, 102, 10047-10056. NDRL 4080
223. Photoelectrochemical behavior of Bi2S3 nanoclusters and nanostructured thin films. Saurez, R.; Nair, P. K.; Kamat, P. V., Langmuir 1998, 14, 3236-3241. NDRL 4048
222. Reaction pathways and kinetic parameters of sonolytically induced oxidation of dimethyl methylphosphonate in air saturated aqueous solutions. O'Shea, K.; Aguila, A.; Vinodgopal, K.; Kamat, P. V., Res. Chem. Intermed. 1998, 24, 695-705. NDRL 4023
221. Picosecond dynamics of Silver nanoclusters. Photoejection of electrons and fragmentation. Kamat, P. V.; Flumiani, M.; Hartland, G., J. Phys. Chem. B 1998, 102, 3123-3128. NDRL 4039
220. Ultrasonic mineralization of reactive textile azo dye, Remazol Black B. Vinodgopal, K.; Peller, J.; Makogon, O.; Kamat, P. V., Water Research 1998, 32, 3646-3650. NDRL 4034
219. Excited State Behavior of Larger Fullerenes, C76 and C78. Prashant V. Kamat and Dirk M. Guldi, Proceedings of the Electrochemical Society. Fullerene Vol.5
218. Role of Iodide in Photoelectrochemical Solar Cells. Electron Transfer between Iodide Ions and Ruthenium Polypyridyl Complex Anchored on Nanocrystalline SiO2 and SnO2 Films. Nasr, C.; Hotchandani, S.; Kamat, P. V., J. Phys. Chem. B 1998, 102, 4944-4951. NDRL 4032
217. Radiation induced reactions of 2,4,6-trinitrotoluene in aqueous solution. Schmelling, D. C.; Gray, K. A.; Kamat, P. V., Environ. Sci. Technol. 1998, 32, 971-974. NDRL 4021
216. Photosensitization of composite semiconductor based nanocrystalline semiconductor films. I. Bedja, S. Hotchandani, P. V. Kamat, Ber. Bunsenges. Phys. Chem.
215. Excited state interactions in pyrrolidinofullerenes. George Thomas, K.; Biju, V.; George, M. V.; Guldi, D. M.; Kamat, P. V., J. Phys. Chem. A 1998, 102, 5341-5348. NDRL 4018
214. Radiolytic reduction and oxidation of diethyl benzylphosphonate. A pulse radiolysis study. A. Aguila, E. O'Shea, and P. V. Kamat, Advance Oxidation Technology 1998, 3 37-42. NDRL 4008
213. Electron Transfer Processes in Nanostructured Semiconductor Thin Films. Kamat, P. V., In Nanoparticles and Nanostructural Films, J. Fendler, Ed.; 1998, Wiley-VCH: New York. p. 207-233.
1997
212. Environmental Photochemistry with Semiconductor Nanoparticles. P. V. Kamat and K. Vinodgopal, In Molecular and Supramolecular Photochemistry, Vol. 2 V. Ramamurthy, Ed.; Marcel Dekker, New York, 1997, p. in press. NDRL 4016
211. Photoelectrochemical behavior of composite semiconductor thin films and their sensitizaton with ruthenium polypyridyl complex. C. Nasr, S. Hotchandani, and P. V. Kamat, In Photoelectrochemistry, K. Rajeshwar, Ed.; The Electrochemical Socity, Pennington, N. J., 1997, 150
210. Excited State Behavior of Fullero-phenylpyrrolidines. P. V. Kamat, D. M. Guldi, D. Liu, K. George Thomas, V. Biju, D. S., and M. V. George, In Fullerenes, Vol. 4, K. Kadish and R. Ruoff, Ed.; The Electrochemical Socity, Pennington, N. J., 1997, p. 122.
209. Recent Developments in Photoexcited States and Reactive Intermediates of Fullerene and Fullerene-Based Supramolecular Assemblies. D. M. Guldi and P. V. Kamat, In Fullerenes, Vol. 4 K. Kadish and R. Ruoff, Ed.; The Electrochemical Socity, Pennington, N. J., 1997, p. 2.
208. Ultrafast study of interfacial electron transfer between 9-anthracene-carboxylate and TiO2 semiconductor particles. I. Martini, G. Hartland, and P. V. Kamat, J. Chem. Phys. 1997, 107, 8064. NDRL 4000
207. Excited, Reduced and Oxidized forms of C76(2n') and C78(D2). D. Guldi, D. Liu, and P. V. Kamat, J. Phys. Chem. 1997, 101, 6195-6201. NDRL 3994
206. Interparticle electron transfer in metal/semiconductor composites. Picosecond dynamics of CdS capped gold nanoclusters. B. Shanghavi and P. V. Kamat, J. Phys. Chem.B 1997, 101, 7675. NDRL 3990
205. Photoelectrochemistry of composite semiconductor thin films. Photosensitization of SnO2/CdS coupled nanocrystallites with a Ruthenium complex. C. Nasr, S. Hotchandani, W. Y. Kim, R. H. Schmehl, and P. V. Kamat, J. Phys. Chem.B 1997, 101, 7480-7487. NDRL 3989
204. Transient absorption spectroscopy of semiconductor nanoclusters. P. V. Kamat, Rev. Laser Eng. 1997, 25, 417. NDRL 3987
203. Photosensitization of Nanocrystalline ZnO Films by Bis(2,2'-bipyridine)(2,2'-bipyridine-4,4'-dicarboxylic acid)ruthenium(II). I. Bedja, P. V. Kamat, X. Hua, A. G. Lappin, and S. Hotchandani, Langmuir 1997, 13, 2398. NDRL 3986
202. Photostabilization of organic dyes on polystyrene capped TiO2 nanoparticles. L. Ziolkowski, K. Vinodgopal, and P. V. Kamat, Langmuir 1997, 13, 3124. NDRL 3981
201. Picosecond Dynamics of an IR sensitive Squaraine Dye. Role of Singlet and Triplet Excited States in the Photosensitization of TiO2 Nanoclusters. D. Liu, P. V. Kamat, K. George Thomas, K. J. Thomas, S. Das, and M. V. George, J. Chem. Phys. 1997, 106(15), 6404-6411. NDRL 3973
200. Semiconductor Nanoparticles TSRP News Letter, SR 180
199. Ultrafast Investigation of the Photophysics of Cresyl Violet adsorbed onto Nanometer Sized Particles of SnO2 and SiO2. I. Martini, G. Hartland, and P. V. Kamat, J. Phys. Chem 1997, 101, 4826. NDRL 3967
198. Hydroxyl radical mediated oxidation: A common pathway in the photocatalytic, radiolytic and sonolytic degradation of textile azo dyes. K. Vinodgopal and P. V. Kamat, In Environmental Applications of Ionizing RadiationW. J. Cooper, R. Curry, and K. O'Shea, Ed.; German Trailer, 1997. NDRL 3966
197. Photocatalytic Reduction of Azo Dyes Naphthol Blue Black and Disperse Blue 79. C. Nasr, K. Vinodgopal, S. Hotchandani, A. K. Chattopadhyay, and P. V. Kamat, Res. Chem. Intermed. 1997 23, 219. NDRL 3965
196. Photoelectrochemical behavior of coupled SnO2/CdSe nanocrystalline semiconductor films. Nasr, P. V. Kamat and S. Hotchandani, J. Electroanal. Chem. 1997,420, 201-207. NDRL 3963
195. Capped Semiconductor Colloids: Synthesis and Photochemistry of CdS Capped SnO2 Nanocrystallites. R. Kennedy, I. Martini, G. Hartland, and P. Kamat, Solar Energy Mat. Solar Cells 1997, 109, 6, 497-507. NDRL 3961
194. Dye Capped Semiconductor Nanoclusters. Role of Back Electron Transfer in the Photosensitization of SnO2 Nanocrystallites with Cresyl Violet Aggregates. D. Liu, R.W. Fessenden, G.L. Hug, and P.V. Kamat, J. Phys. Chem. B 1997, 101, 2583. NDRL 3960
193. Semiconductor Nanoclusters-Physical, Chemical and Catalytic Aspects. BOOK : P. V. Kamat and D. Meisel, eds. Studies in Surface Science and Catalysis, Elsevier Science, Amsterdam, 1997.
192. Photocatalytic degradation of 4-chlorophenol. 2. A. Model. U. Stafford, K. A. Gray, and P. V. Kamat, Res. Chem. Intermed. 1997, 23, 355. NDRL 3786
191. Photocatalytic degradation of 4-chlorophenol. 1. The effects of varying TiO2 concentration and light wavelength. U. Stafford, K. A. Gray, and P. V. Kamat, J. Catal. 1997, 167, 25. NDRL 3785
190. Radical reactions of C84. P. V. Kamat, G. Sauve, D. M. Guldi, and K.-D. Asmus, Res. Chem. Intermed. 1997 23, 575. NDRL 3762
189. Photochemistry on semiconductor surfaces. Photochemical oxidation of C60 on TiO2 nanoparticles. P. V. Kamat, M. Gevaert, and K. Vinodgopal, J. Phys. Chem. B 1997, 101(22), 4422–4427. NDRL 3944
188. Excited states and reduced and oxidized forms of a textile diazo dye, naphthol blue black. Spectral characterization using laser flash photolysis and pulse radiolysis studies. C. Nasr, K. Vinodgopal, S. Hotchandani, and P. V. Kamat, Rad. Phys. Chem. 1997, 49, 159. NDRL 3927
187. The influence of solution matrix on the photocatalytic degradation of TNT in TiO2 slurries. D.C. Schmelling, K.A. Gray, and P.V. Kamat, Water Research 1997, 31(6), 1439–1447. NDRL 3926
186. Excited states and reduced and oxidized forms of a textile diazo dye, naphthol blue black. Spectral characterization using laser flash photolysis and pulse radiolysis studies. C. Nasr, K. Vinodgopal, S. Hotchandani, and P. V. Kamat, Rad. Phys. Chem. 1997, 49, 159. NDRL 3927
185. The influence of solution matrix on the photocatalytic degradation of TNT in TiO2 slurries. D.C. Schmelling, K.A. Gray, and P.V. Kamat, Water Research 1997, 31(6), 1439–1447. NDRL 3926
184. Native and surface modified semiconductor nanoclusters. P.V. Kamat, In Molecular level artificial photosynthetic materials. Progress in Inorganic Chemistry Series. J. Meyer, Editor. 1997, John Wiley & Sons, Inc.: New York. p. 273-243. NDRL 3808
1996
183. Photochemistry of squaraine dyes. 10. Excited state properties and photosensitization behavior of an IR sensitive cationic squaraine dye. K. George Thomas, K.J. Thomas, S. Das, M.V. George, D. Liu, and P.V. Kamat, Faraday Trans. 1996, 92(24) 4913-4916. NDRL 3950
182. Sonochromic Effect in WO3 Colloidal Suspensions. P.V. Kamat and K. Vinodgopal, Langmuir 1996, 12(23), 5739-41. NDRL 3943
181. Fluorescence and photoelectrochemical behavior of chlorophyll a adsorbed on a nanocrystalline SnO2 film. I. Bedja, P.V. Kamat, and S. Hotchandani, J. Appl. Phys. 1996, 80(8), 4637-43. NDRL 3939
180. Recent developments in photoexcited fullerenes and charge transfer interactions. In Fullerenes: Chemistry, Physics, and New Directions P.V. Kamat and D. Guldi, R. Ruoff and K. Kadish, Ed.; Fullerenes, Vol. 3, 1996, Electrochemical Society: Pennington, New Jersey. p. 254-263. SR179
179. Photochemical behavior of C60 on TiO2 surface. In Fullerenes: Chemistry, Physics, and New Directions P.V. Kamat, M. Geavert, and K. Vinodgopal, R. Ruoff and K. Kadish, Ed.; Fullerenes, Vol. 3, 1996, Electrochemical Society: Pennington, New Jersey. p. 376-383. NDRL 3938
178. Composite Semiconductor Nanoclusters. P.V. Kamat, In Nanocrystalline semiconductor materials, P.V. Kamat and D. Meisel, Ed.; 1996, Elsevier Science: Amsterdam. NDRL 3928
177. Photochemical Solar Cells. A successful marriage between semiconductor nanoclusters and excited dyes. P.V. Kamat, IAPS Newsletter 1996, 19, 14-23. SR 178
176. Photoexcited Fullerenes P.V. Kamat and K.-D. Asmus, Interface 1996, 5, 22-25.
175. Dye-Capped Semiconductor Nanoclusters. Excited State and Photosensitization Aspects of Rhodamine 6G H-Aggregates Bound to SiO2 and SnO2 Colloids. C. Nasr, D. Liu, S. Hotchandani, and P. Kamat, J. Phys. Chem. 1996, 100, 11054-11061. NDRL 3900
174. Dye-Capped Semiconductor Nanoclusters. One-Electron Reduction and Oxidation of Thionine and Cresyl Violet H-Aggregates Electrostatically Bound to SnO2 Colloids. D. Liu and P.V. Kamat, Langmuir 1996, 12, 2190-2195. NDRL 3895
173. Environmental Photochemistry on Semiconductor Surfaces. Visible Light Induced Degradation of A Textile Diazo Dye, Naphthol Blue Black on TiO2 particles. C. Nasr, K. Vinodgopal, S. Hotchandani, A. Chattopadhyaya, and P.V. Kamat, J. Phys. Chem. 1996, 100, 8436-8442. NDRL 3894
172. Role of Reduction in the Photocatalytic Degradation of TNT. D. Schmelling, K.A. Gray, and P.V. Kamat, Environ. Sci. Technol. 1996, 30, 2547-2555. NDRL 3893
171. Combine Electrochemistry with Photocatalysis. K. Vinodgopal and P.V. Kamat, CHEMTECH 1996, April, 18-22. NDRL 3892
170. Picosecond dynamics of Cresyl violet H-aggregates adsorbed on SiO2 and SnO2 nanocrystallites. D. Liu and P.V. Kamat, J. Chem. Phys. 1996, 105, 965-970. NDRL 3881
169. Environmental Photochemistry on Semiconductor Surfaces: Photosensitized Degradation of a Textile Azo Dye, Acid Orange 7, on TiO 2 Particles Using Visible Light7. K. Vinodgopal, D. Wynkoop, and P.V. Kamat, Environ. Sci. Technol. 1996, 30, 1660-1666. NDRL 3868
168. Aggregation Behavior of Water Soluble Bis(benzothiazolylidene)squaraine Derivatives in Aqueous Media. S. Das, J. Thomas, K.G. Thomas, V. Madhavan, D. Liu, P.V. Kamat, and M.V. George, J. Phys. Chem. 1996, 100, 17287-17296. NDRL 3855
167. Photochemistry of squaraine dyes: Excited triplet state and redox properties of crown ether squaraines. G. Sauve, P.V. Kamat, K.G. Thomas, J. Thomas, S. Das, and M.V. George, J. Phys. Chem. 1996, 100, 2117-2124. NDRL 3854
166. Photoinduced charge transfer processes in semiconductor heterostructures. Capped vs. Coupled systems. P.V. Kamat and K. Vinodgopal, In Fine Particles Science and Technology: From Micro to Nanoparticles, E. Pelizzetti, Ed.; 1996, Kluwer Academic Publishers: Dordrecht, The Netherlands. p. 303-316. NDRL 3827
165. Nanostructured Semiconductor Films for Photocatalysis. Photoelectrochemical Behavior of SnO2/TiO2 Composite Systems and Its Role in Photocatalytic Degradation of a Textile Azo Dye. K. Vinodgopal, I. Bedja, and P.V. Kamat, Chem. Mater. 1996, 8, 2180. NDRL 3837
164. Photosensitization of Nanocrystalline Semiconductor Films. Modulation of Electron Transfer between Excited Ruthenium Complex and SnO2 Nanocrystallites with an Externally Applied Bias. P.V. Kamat, I. Bedja, S. Hotchandani, and L.K. Patterson, J. Phys. Chem. 1996, 100, 4900-4908. NDRL 3830
163. Transient absorption spectroscopy of nanostructured semiconductor films at controlled potentials. An in situ spectroelectrochemical investigation of the photosensitization process. I. Bedja, S. Hotchandani, and P.V. Kamat, J. Electroanal. Chem. 1996, 401, 237-241. NDRL 3812
162. Photocatalytic degradation of organic contaminants. Halophenols and related model compounds. U. Stafford, K.A. Gray, and P.V. Kamat, Heterogeneous Chemistry Reviews 1996, 3, 77-104. NDRL 3801
1995
161. Photochemistry on Surfaces. Intermolecular energy and electron transfer processes between excited Ru(bpy)32+ and H-aggregates of Cresyl Violet on SiO2 and SnO2 colloids. D. Liu, G.L. Hug, and P.V. Kamat, J. Phys. Chem. 1995, 99, 16768-16775. NDRL 3846
160. Photoexcited and charge transfer processes - An Overview. In Recent Advances in the Chemistry and Physics of Fullerenes and Related Materials P.V. Kamat and K.-D. Asmus, K.M. Kadish and R.R. S., Ed.; 1995, The Electrochemical Society, Inc.: Pennington, N.J., U.S.A. p. 386-398.
159. Photophysical and charge transfer processes of C84. In Recent Advances in the Chemistry and Physics of Fullerenes and Related Materials. G. Sauve and P.V. Kamat, K.M. Kadish and R.R. S., Ed. 1995, The Electrochemical Society, Inc.: Pennington, N.J., U.S.A. p. 399-405.
158. Tailoring Nanostructured Semiconductor Thin Films. P.V. Kamat, Chemtech 1995, June, 22-28. NDRL 3800
157. Rate constants for charge injection from excited sensitizer into SnO2, ZnO, and TiO2 semiconductor nanocrystallites. R.W. Fessenden and P.V. Kamat, J. Phys. Chem. 1995, 99, 12902-12906. NDRL 3799.
156. CdSe/SnO2 coupled semiconductor films. Electrochemical and photoelectrochemical studies. C. Nasr, S. Hotchandani, and P.V. Kamat, Proc. Ind. Acad. Sci. 1995, 107, 699-708. NDRL 3787.
156. Capped semiconductor colloids. Synthesis and Photoelectrochemical properties of TiO2 capped SnO2 surfaces. I. Bedja and P.V. Kamat, J. Phys. Chem. 1995, 99, 9182-9188. NDRL 3783.
155. Photochemistry of Ru(bpy)2(dcbpy)2+ on Al2O3 and TiO2 surfaces. An insight into the mechanism of photosensitization. K. Vinodgopal, X. Hua, R.L. Dahlgren, A.G. Lappin, L.K. Patterson, and P.V. Kamat, J. Phys. Chem. 1995, 99, 10883-10889. NDRL 3781.
154. Electrochemical and photoelectrochemical properties of monoaza-15-Crown ether linked cyanine dyes: Photosensitization of nanocrystalline SnO2 films. C. Nasr, S. Hotchandani, P.V. Kamat, S. Das, K. George Thomas, and M.V. George, Langmuir 1995, 11, 1777-1783.
153. Electrochemically assisted photocatalysis using nanocrystalline semiconductor films. K. Vinodgopal and P.V. Kamat, Solar Energy Mater. Solar Cells 1995, 38, 401-410.
152. Singlet and triplet excited state behaviors of C60 in nonreactive and reactive polymer films. G. Sauve, N.M. Dimitrijevic, and P.V. Kamat, J. Phys. Chem. 1995, 99, 1199-203. NDRL 3761
151. Photophysical and Photoelectrochemical Behavior of Poly[styrene-co-3-(acrylamido)-6-aminoacridine]. S. Das, C.S. Rajesh, C.H. Suresh, K.G. Thomas, A. Ajayaghosh, C. Nasr, P.V. Kamat, and M.V. George, Macromolecules 1995, 28, 4249-4254. NDRL 3755.
150. Enhanced rates of photocatalytic degradation of an azo dye using SnO2/TiO2 coupled semiconductor thin films. K. Vinodgopal and P.V. Kamat, Environ. Sci. Technol. 1995, 29, 841-845. NDRL 3750
149. Excited triplet and reduced forms of C84. G. Sauve, P.V. Kamat, and R.S. Ruoff, J. Phys. Chem. 1995 99, 2162-5. NDRL 3734
148. Crown ether derivatives of squaraine. New near infrared-absorbing, redox-active fuoroionophores for alkali metal recognition. S. Das, K.G. Thomas, J. Thomas, M.V. George, I. Bedja, and P.V. Kamat, Anal. Proc. 1995, 32, 213-215. NDRL 3621
147. Electrochemically active nanocrystalline SnO2 films. Surface modification with thiazine and oxazine dye aggregates. D. Liu and P.V. Kamat, J. Electrochem. Soc. 1995, 142, 835-839.
146. Photocatalytic oxidation of 4-chlorophenol on TiO2: A comparision with g-radiolysis. U. Stafford, K.A. Gray, and P.V. Kamat, In Chemical Oxidation: Technologies for the 90's, J. Roth and A. Bowers, Editors. 1995, Technomic Publishing Co.: Lancaster, Pennsylvania. p. 193-204. NDRL 3676
1994
145. The sensitized photocatalysis of a mixed reactant system of 4-chlorophenol and 4-nitrophenol. M.S. Dieckmann, K.A. Gray, and P.V. Kamat, In Conference on Environmental Engineering, Critical Issues in Water and Wastewater Treatment. 1994: American, Society of Civil Engineers, Boulder, Colorado.
144. Interfacial charge transfer processes in colloidal semiconductor systems. P.V. Kamat, Progr. React. Kinetics 1994, 19, 277-316. SR 164
143. Photoinduced charge transfer between fullerenes and TiO2 Semiconductor colloids. P.V. Kamat, I. Bedja, and S. Hotchandani, In Recent Advances in the Chemistry and Physics of Fullerenes and Related Materials, K.M. Kadish and R.R. S., Editors. 1994, The Electrochemical Society, Inc.: Pennington, N.J., U.S.A. p. 964-975. NDRL 3707
142. Semiconductor particulate films for the photocatalytic degradation of organic contaminants. K. Vinodgopal, U. Stafford, K.A. Gray, and P.V. Kamat. In Water Purif. Photocatal., Photoelectrochem., Electrochem. Processes, 1994: The Electrochemical Society. NDRL 3708
141. Photochemistry of squaraine dyes. 8. Photophysical properties of crown ether squaraine fluoroionphores and their metal ion complexes. S. Das, K.G. Thomas, J. Thomas, M.V. George, and P.V. Kamat, J. Phys. Chem. 1994, 98, 9291-9296.
140. Photoinduced charge transfer between carbon and semiconductor clusters. One-electron reduction of C60 in colloidal TiO2 Semiconductor suspensions. P.V. Kamat, I. Bedja, and S. Hotchandani, J. Phys. Chem. 1994, 98, 9137-9142. NDRL 3681
139. Photochemistry of textile azo dyes. Spectral characterization of excited state, reduced and oxidized forms of Acid Orange 7. K. Vinodgopal and P.V. Kamat, J. Photochem. Photobiol., A:Chemistry 1994, 83, 141-146.
138. Radiolytic and TiO2 assisted photocatalytic degradation of 4-chlorophenol. A comparative study. U. Stafford, K.A. Gray, and P.V. Kamat, J. Phys. Chem. 1994, 98, 6343-6351. NDRL 3669
137. Preparation and characterization of thin SnO2 nanocrystalline semiconductor films and their sensitization with bis(2,2'-bipyridine)(2,2'-bipyridine-4-4'-dicarboxylic acid)ruthenium complex. I. Bedja, S. Hotchandani, and P.V. Kamat, J. Phys. Chem. 1994, 98, 4133-4140. NDRL 3662
136. Chlorophyll b modified nanocrystalline SnO2 semiconductor thin film as a photosensitive electrode. I. Bedja, S. Hotchandani, R. Carpentier, R.W. Fessenden, and P.V. Kamat, J. Appl. Phys. 1994, 75, 5444-5456. NDRL 3661 NDRL 3661
135. Interaction of semiconductor colloids with J-aggregates of squaraine dye and its role in sensitizing nanocrystalline semiconductor films. S. Hotchandani, S. Das, K.G. Thomas, M.V. George, and P.V. Kamat, Res. Chem. Intermed. 1994, 20, 927-938. NDRL 3653
134. A photocatalytic approach for the reductive decolorization of textile azo dyes in colloidal semiconductor suspensions. K. Vinodgopal, I. Bedja, S. Hotchandani, and P.V. Kamat, Langmuir 1994, 10, 1767-1771. NDRL 3652
133. Electrochemically Assisted Photocatalysis. 2. The Role of Oxygen and Reaction Intermediates in the Degradation of 4-Chlorophenol on Immobilized TiO2 Particulate Films. K. Vinodgopal, U. Stafford, K.A. Gray, and P.V. Kamat, J. Phys. Chem. 1994, 98, 6797-6803. NDRL 3645
132. Electrochromic and photoelectrochemical behavior of thin WO3 films prepared from quantized colloidal particles. I. Bedja, S. Hotchandani, R. Carpentier, K. Vinodgopal, and P.V. Kamat, Thin Solid Films 1994, 247, 195-200. NDRL 3636
131. Mechanistic studies of chloro- and nitrophenolic degradation on semiconductor surfaces. K.A. Gray, P.V. Kamat, U. Stafford, and M. Dieckmann, In Aquatic and Surface Photochemistry, G.R. Helz, R.G. Zepp, and D.G. Crosby, Ed.; 1994, CRC Press, Inc.: Boca Raton, Fl. p. 399-408. NDRL 3451
130. Photosensitization of semiconductor colloids by humic substances. K. Vinodgopal and P.V. Kamat, In Aquatic and Surface Photochemistry, G.R. Helz, R.G. Zepp, and D.G. Crosby, Ed.; 1994, CRC Press, Inc.: Boca Raton, Fl. p. 437-442. NDRL 3450
129. The role of support material in the photodegradation of colored organic compounds. P.V. Kamat and K. Vinodgopal, In Aquatic and Surface Photochemistry, G.R. Helz, R.G. Zepp, and D.G. Crosby, Ed.; 1994, CRC Press, Inc.: Boca Raton, Fl. p. 443-450. NDRL 3435
128. Electrochromic and photoelectrochromic behavior of thin WO3 films prepared from quantum size colloidal particles. S. Hotchandani, I. Bedja, R.W. Fessenden, and P.V. Kamat, Langmuir 1994, 10, 17-22. NDRL 3549
1993
127. What makes semiconductor colloids unique as photocatalysts. P.V. Kamat, Spectrum 1993, 6, 14-20. NDRL 3657
126. Photochemistry of squaraine dyes. 6. Solvent hydrogen bonding effects on the photophysical properties of bis(benzothiazolylidene)squaraines. S. Das, K.G. Thomas, R. Ramanathan, M.V. George, and P.V. Kamat, J. Phys. Chem. 1993, 97, 13625-8. NDRL 3641
125. Photochemistry of squaraine dyes. 5. Aggregation of bis(2,4-dihydroxyphenyl)squaraine and bis(2,4,6- trihydroxyphenyl)squaraine and their photodissociation in acetonitrile solutions. S. Das, T.L. Thanulingam, K.G. Thomas, P.V. Kamat, and M.V. George, J. Phys. Chem. 1993, 97, 13620-4.
124 Electrochemically assisted photocatalytic degradation of 4-chlorophenol using TiO2 particulate films. K. Vinodgopal, Hotchandani, and P.V. Kamat. In Envirronmental Aspects of Electrochemistry and Photoelectrochemistry, 1993. Honolulu: The Electrochemical Society. NNDRL 3581
123. Photoelectrochemistry of quantized tungsten trioxide colloids: electron storage, electrochromic, and photoelectrochromic effects. I. Bedja, S. Hotchandani, and P.V. Kamat, J. Phys. Chem. 1993, 97, 11064-70. NDRL 3606
122. Photosensitizing properties of squaraine dyes. S. Das, K.G. Thomas, P.V. Kamat, and M.V. George, Proc. Indian Acad. Sci. (Chem. Sci.) 1993, 105, 513-525. NDRL 3601
121. Photoelectrochemical behavior of thin CdSe and coupled TiO2/CdSe semiconductor films. D. Liu and P.V. Kamat, J. Phys. Chem. 1993, 97, 10769-73. NDRL 3601
120. Aggregates of C60 and C70 formed at the gas-water interface and in DMSO/water mixed solvents. A spectral study. Y.M. Wang, P.V. Kamat, and L.K. Patterson, J. Phys. Chem. 1993, 97, 8793-7. NDRL 3587
119. Excited-state behavior and one-electron reduction of C60 in aqueous gamma-cyclodextrin solution. N.M. Dimitrijevic and P.V. Kamat, J. Phys. Chem. 1993, 97, 7623-6. NDRL 3575
118. Photoinduced charge transfer processes in ultrasmall semiconductor clusters. Photophysical properties of CdS clusters in Nafion membrane. K.R. Gopidas and P.V. Kamat, Proc. Ind. Acad. Sci. (Chem. Sci.) 1993, 105, 505-512. NDRL 3555
117. Mechanistic studies in TiO2 systems: Photocatalytic degradation of chloro- and nitrophenols. K.A. Gray, U. Stafford, M.S. Dieckmann, and P.V. Kamat, In Photocatalytic Purification and Treatment of Water and Air, D.F. Ollis and H. Al-Ekabi, Ed.; 1993, Elsevier Science: Amsterdam. p. 455-472. NDRL 3568
116. Electrochemically assisted photocatalysis. TiO2 particulate film electrodes for photocatalytic degradation of 4-chlorophenol. K. Vinodgopal, S. Hotchandani, and P.V. Kamat, J. Phys. Chem. 1993, 97, 9040-4. NDRL 3556
115. TiO2 mediated photocatalysis using visible light: Photosensitization approach. P.V. Kamat and K. Vinodgopal, In Photocatalytic Purification and Treatment of Water and Air, D.F. Ollis and H. Al-Ekabi, Ed.; 1993, Elsevier Science Publishers B.V.: Amsterdam, The Netherlands. p. 83-94.
114. Photochemistry of squaraine dyes. 4. Excited-state properties and photosensitization behaviour of bis(2,4-dihydroxyphenyl)squaraine. P.V. Kamat, S. Hotchandani, M. de Lind, K.G. Thomas, S. Das, and M.V. George, J. Chem. Soc., Faraday Trans. 1993, 89, 2397-402. NDRL 3552
113. Radical adducts of fullerenes C60 and C70 studied by laser flash photolysis and pulse radiolysis. N.M. Dimitrijevic, P.V. Kamat, and R.W. Fessenden, J. Phys. Chem. 1993, 97, 615-8. NDRL 3534
112. Electrochemical rectification in CdSe + TiO2 coupled semiconductor films. D. Liu and P.V. Kamat, J. Electroanal. Chem. Interfacial Electrochem. 1993, 347, 451-6. NDRL 3538
110. A new photodegradable polyamide containing o- nitrobenzyl chromophore. Steady state and laser flash photolysis studies. T. Mathew, A. Ajayaghosh, S. Das, P.V. Kamat, and M.V. George, J. Photochem. Photobiol., A 1993, 71, 181-9. NDRL 3526
109. Photocatalyzed degradation of adsorbed nitrophenolic compounds on semiconductor surfaces. M.S. Dieckmann, K.A. Gray, and P.V. Kamat, Wat. Sci. Tech. 1992, 25, 277-279. NDRL 3528
108. Photophysics and photochemistry of squaraine dyes. 3. Excited-state properties and poly(4-vinylpyridine)- induced fluorescence enhancement of bis(2,4,6- trihydroxyphenyl)squaraine. S. Das, P.V. Kamat, B. de la Barre, K.G. Thomas, A. Ajayaghosh, and M.V. George, J. Phys. Chem. 1992, 96, 10327-30. NDRL 3517
107. Quenching of fullerene triplets by stable nitroxide radicals. A. Samanta and P.V. Kamat, Chem. Phys. Lett. 1992, 199, 635-9.
106. An in situ diffuse reflectance FTIR investigation of photocatalytic degradation of 4-chlorophenol on a TiO2 powder surface. U. Stafford, K.A. Gray, P.V. Kamat, and A. Varma, Chem. Phys. Lett. 1993, 205, 55-61. NDRL 3461
105. Surface modification of CdS colloids with mercaptoethylamine. P.V. Kamat, M. de Lind, and S. Hotchandani, Isr. J. Chem. 1993, 33, 47-51.
104. Photocrosslinking studies of S-acryloyl O-ethyl xanthate copolymers. A. Ajayaghosh, S. Das, P.V. Kamat, P.K. Das, and M.V. George, Polymer 1993, 34, 3605-10.
103. GaAs quantum dots. A.J. Nozik, H. Uchida, P.V. Kamat, and C.J. Curtis, Israel J. Chem. 1993, 33, 15-20.
102. Environmental photochemistry on surfaces. Charge injection from excited fulvic acid into semiconductor colloids. K. Vinodgopal and P.V. Kamat, Environ. Sci. Technol. 1993, 26, 1963-6. NDRL 3441
1992
101. Fluorescence enhancement of bis(2,4,6- trihydroxyphenyl)squaraine anion by 2:1 host-guest complexation with beta- cyclodextrin. S. Das, K.G. Thomas, M.V. George, and P.V. Kamat, J. Chem. Soc. 1992, Faraday Trans, 88, 3419-22. NDRL 3514
100. Photochemistry of fullerenes. Excited-state behavior of C60 and C70 and their reduction in poly(methyl methacrylate) films. M. Gevaert and P.V. Kamat, J. Phys. Chem. 1992, 96, 9883-8. NDRL 3500
99. Visible laser-induced oxidation of C70 on titanium dioxide particles. M. Gevaert and P.V. Kamat, J. Chem. Soc. 1992, Chem. Commun., 1470-2. NDRL 3496
98. Charge-transfer processes in coupled semiconductor systems. Photochemistry and photoelectrochemistry of the colloidal CdS-ZnO system. S. Hotchandani and P.V. Kamat, J. Phys. Chem. 1992, 96, 6834-9. NDRL 344
97. Photophysics and photochemistry of quantized ZnO colloids. P.V. Kamat and B. Patrick, J. Phys. Chem. 1992, 96, 6829-34. NDRL 3448
96. Triplet excited state behavior of fullerenes: Pulse radiolysis and laser flash photolysis of C60 and C70 in benzene. N.M. Dimitrijevic and P.V. Kamat, J. Phys. Chem. 1992, 96, 4811-4814. NDRL 3438
95. Modification of electrode surface with semiconductor colloids and its sensitization with chlorophyll a. S. Hotchandani and P.V. Kamat, Chem. Phys. Lett. 1992, 191, 320-326. NDRL 3433
94. Photoelectrochemistry of semiconductor ZnO particulate films. S. Hotchandani and P.V. Kamat, J. Electrochem. Soc. 1992, 139, 1630-1634. NDRL 3428
93. Photochemistry of squaraine dyes. 2. Excited states and reduced and oxidized forms of 4-(4-acetyl-3,5- dimethylpyrrolium-2-ylidene)-2-(4-acetyl-3,5- dimethylpyrrol-2-yl)-3-oxocyclobut-1-en-1-olate. B. Patrick, M.V. George, P.V. Kamat, S. Das, and K.G. Thomas, J. Chem. Soc., Faraday Trans 1992, 88, 671-676. NDRL 3419
92. Optical properties of GaAs nanocrystals. H. Uchida, C.J. Curtis, P.V. Kamat, K.M. Jones, and A.J. Nozik, J. Phys. Chem. 1992, 96, 1156-1160.
91. Photoelectrochemistry in semiconductor particulate systems. Part 17. Photosensitization of large-bandgap semiconductors. Charge injection from triplet excited thionine into ZnO colloids. B. Patrick and P.V. Kamat, J. Phys. Chem. 1992, 96, 1423-1428. NDRL 3403
90. Photochemistry on surfaces. Photodegradation of 1,3- diphenylisobenzofuran over metal oxide particles. K. Vinodgopal and P.V. Kamat, J. Phys. Chem. 1992, 96, 5053-5059. NDRL 3402
89. Sensitized charge injection in large-band-gap semiconductor colloids. P.V. Kamat and B. Patrick, In Electrochemistry in Colloids and Dispersions, R.A. Mackay and J. Texter, Ed.; 1992, VCH Publishers: New York. p. 447-455. NDRL 3390
88. Photochemistry of squaraine dyes. 1. Excited singlet, triplet, and redox states of bis[4- (dimethylamino)phenyl]squaraine and bis[4-(dimethylamino)-2-hydroxyphenyl]squaraine. P.V. Kamat, S. Das, K.G. Thomas, and M.V. George, J. Phys. Chem. 1992, 96, 195-9. NDRL 3368
87. Photochemistry on surfaces. Excited state behavior of ruthenium tris(bathophenanthroline disulfonate) on colloidal alumina-coated silica particles. P.V. Kamat and W.E. Ford, Photochem. Photobiol. 1992, 55, 159-63. NDRL 3359
86. Photochemistry on surfaces. Photochemical behavior of 1,3-diphenylisobenzofuran over alumina. K. Vinodgopal and P.V. Kamat, J. Photochem. Photobiol., A 1992, 63, 119-25. NDRL 3355
1991
85. Photoinduced charge transfer between fullerenes (C60 and C70) and semiconductor ZnO colloids. P.V. Kamat, J. Am. Chem. Soc. 1991, 113, 9705-7. NDRL 3405
84. Photochemistry and photophysics of ZnO colloids. P.V. Kamat and B. Patrick. In Symp. Electron. Ionic Prop. Silver Halides, 1991. Springfield, Va: The Society for Imaging Science and Technology. NDRL 3349
83. Ultrafast photochemical events associated with the photosensitization properties of a squaraine dye. P.V. Kamat, S. Das, K.G. Thomas, and M.V. George, Chem. Phys. Lett. 1991, 178, 75-9. NDRL 3314
82. Photochemistry of sensitizing dyes. Spectroscopic and redox properties of cresyl violet. D.I. Kreller and P.V. Kamat, J. Phys. Chem. 1991, 95, 4406-10. NDRL 3313
81. Photoelectrochemical sensitization and spectroscopic properties of reduced and oxidized forms of a chlorophyll analogue. P.V. Kamat and J.P. Chauvet, Radiat. Phys. Chem. 1991, 37, 705-9. NDRL 3272
80. Photophysics, photochemistry, and photocatalytic aspects of semiconductor clusters and colloids. P.V. Kamat, In Kinetics and Catalysis in Microheterogeneous Systems, M. Graetzel and K. Kalyanasundaram, Editors. 1991, Marcel Dekker, Inc.: New York. p. 375-436. SR135
79. Spectral differences between enantiomeric and racemic Ru(bpy)32+ on layered clays: Probable causes. P.V. Kamat, K.R. Gopidas, T. Mukherjee, V. Joshi, D. Kotkar, V.S. Pathak, and P.K. Ghosh, J. Phys. Chem. 1991, 95, 10009-18.
78. Electron transfer reactions. Reaction of tetracyclone, tetraphenylfuran and related substrates with potassium. B. Pandey, M.P. Mahajan, R.K. Tikare, M. Muneer, N.P. Rath, P.V. Kamat, and M.V. George, Res. Chem. Intermed. 1991, 15, 271-91.
1990-1989
77. Charge transfer processes in semiconductor colloids. P.V. Kamat and K.R. Gopidas. In Picosecond and Femtosecond Spectroscopy from Laboratory to Real World, 1990. Los Angeles: SPIE-Int. Soc. Opt. Eng.
76. Photochemical processes on oxide surfaces. A diffuse reflectance laser flash photolysis study. K.R. Gopidas, P.V. Kamat, and M.V. George, Mol. Cryst. Liq. Cryst. 1990, 183, 403-9.
75. Picosecond charge transfer processes in ultrasmall CdS and CdSe semiconductor particles. P.V. Kamat, K.R. Gopidas, and N.M. Dimitrijevic, Mol. Cryst. Liq. Cryst. 1990, 183, 439-45.
74. Photoelectrochemistry in semiconductor particulate systems. 16. Photophysical and photochemical aspects of coupled semiconductors: charge-transfer processes in colloidal cadmium sulfide-titania and cadmium sulfide-silver(I) iodide systems. K.R. Gopidas, M. Bohorquez, and P.V. Kamat, J. Phys. Chem. 1990, 94, 6435-40.
73. Electron transfer reactions. Reaction of dibenzobarrelenes with potassium. K. Ashok, P.V. Kamat, and M.V. George, Res. Chem. Intermed. 1990, 13, 203-20.
72. Photoelectrochemistry in semiconductor particulate systems. 15. Photophysical behavior of ultrasmall CdSe semiconductor particles in a perfluorosulfonate membrane. K.R. Gopidas and P.V. Kamat, Mater. Lett. 1990, 9, 372-8.
71. Photochemistry in polymers. Photoinduced electron transfer between phenosafranine and triethylamine in perfluorosulfonate membrane. K.R. Gopidas and P.V. Kamat, J. Phys. Chem. 1990, 94, 4723-7.
70. Photoelectrochemistry in semiconductor particulate systems. 14. Picosecond charge-transfer events in the photosensitization of colloidal TiO2. P.V. Kamat, Langmuir 1990, 6, 512-3.
69. Colloidal semiconductors as photocatalysts for solar energy conversion. P.V. Kamat and N.M. Dimitrijevic, Sol. Energy 1990, 44, 83-98.
68. Electron transfer reactions. Reactions of epoxyketones and benzoylaziridines with potassium. K. Ashok, R.K. Tikare, P.V. Kamat, and M.V. George, Res. Chem. Intermed. 1990, 13, 117-42.
67. Electron transfer reactions. Reaction of nitrogen heterocycles with potassium. M. Muneer, P.V. Kamat, and M.V. George, Can. J. Chem. 1990, 68, 969-75.
66. Primary photophysical and photochemical processes of dyes in polymer solutions and films. P.V. Kamat and M.A. Fox, In Lasers in Polymer Science and Technology: Applications, J.P. Fouassier and J.F. Rabek, Ed.; 1990, CRC Press: Boca Raton, FL. p. 185-202.
65. Photochemistry of 3,4,9,10-perylenetetracarboxylic dianhydride dyes. 4. Spectroscopic and redox properties of oxidized and reduced forms of the bis(2,5-di-tert- butylphenyl)imide derivative. W.E. Ford, H. Hiratsuka, and P.V. Kamat, J. Phys. Chem. 1989, 93, 6692-6.
64. Excited dyes improve photosensitivity of semiconductors. P.V. Kamat, Research & Development 1989, June, 87-90.
63. Photochemistry on surfaces. 4. Influence of support material on the photochemistry of an adsorbed dye. K.R. Gopidas and P.V. Kamat, J. Phys. Chem. 1989, 93, 6428-33.
62. Dynamic Burstein-Moss shift in semiconductor colloids. P.V. Kamat, N.M. Dimitrijevic, and A.J. Nozik, J. Phys. Chem. 1989, 93, 2873-5.
61. Photophysics and photochemistry of phenosafranin dye in aqueous and acetonitrile solutions. K.R. Gopidas and P.V. Kamat, J. Photochem. Photobiol. 1989, A, 48, 291-301.
60. Photoelectrochemistry in semiconductor particulate systems. 13. Surface modification of CdS semiconductor colloids with diethyldithiocarbamate. P.V. Kamat and N.M. Dimitrijevic, J. Phys. Chem. 1989, 93, 4259-63.
59. Quenching of excited doublet states of organic radicals by stable radicals. A. Samanta, K. Bhattacharyya, P.K. Das, P.V. Kamat, D. Weir, and G.L. Hug, J. Phys. Chem. 1989, 93, 3651-6.
58. Photoelectrochemistry in semiconductor particulate systems. Part 12. Primary photochemical events in CdS semiconductor colloids as probed by picosecond laser flash photolysis. P.V. Kamat, T.W. Ebbesen, N.M. Dimitrijevic, and A.J. Nozik, Chem. Phys. Lett. 1989, 157, 384-9.
57. Photoelectrochemistry in particulate systems. 11. Reduction of phenosafranin dye in colloidal TiO2 and CdS suspensions. K.R. Gopidas and P.V. Kamat, Langmuir 1989, 5, 22-6.
56. Photoelectrochemistry in particulate systems. 9. Photosensitized reduction in a colloidal TiO2 system using anthracene-9-carboxylic acid as the sensitizer. P.V. Kamat, J. Phys. Chem. 1989, 93, 859-64.
55. Photochemistry on surfaces. 3. Spectral and photophysical properties of monomeric and dimeric anthracenesulfonates adsorbed to colloidal alumina- coated silica particles. W.E. Ford and P.V. Kamat, J. Phys. Chem. 1989, 93, 6423-8.
54. Photochemistry on surfaces. 2. Intermolecular electron transfer on colloidal alumina-coated silica particles. P.V. Kamat and W.E. Ford, J. Phys. Chem. 1989, 93, 1405-9.
53. Electron transfer reactions. Reaction of sydnones with potassium. M. Muneer, R.K. Tikare, P.V. Kamat, and M.V. George, New J. Chem. 1989, 13, 215-20.
1988-1986
52. Electron transfer reactions of In2Se3 and In2S3 semiconductor colloids. N.M. Dimitrijevic and P.V. Kamat, Prog. Colloid Polym. Sci. 1988, 76.
51. Photoelectrochemistry in particulate systems. Photosensitized charge injection into opaque TiO2 semiconductor powder as probed by time-resolved diffuse reflectance laser flash photolysis. P.V. Kamat, K.R. Gopidas, and D. Weir, Chem. Phys. Lett. 1988, 149, 491-6.
50. Microphotoelectrolysis with indium sulfide and indium selenide semiconductor colloids. P.V. Kamat and N.M. Dimitrijevic, Proc. Electrochem. Soc. 1988, 6, 90-96.
49. Photoelectrochemistry in particulate systems. 8. Photochemistry of colloidal selenium. N.M. Dimitrijevic and P.V. Kamat, Langmuir 1988, 4, 782-4.
48. Oxidation of In2S3 and In2Se3 colloids as studied by pulse radiolysis. N.M. Dimitrijevic and P.V. Kamat, Radiat. Phys. Chem. 1988, 32, 53-7.
47. Photoelectrochemistry in particulate systems. 7. Electron-transfer reactions of indium sulfide semiconductor colloids. P.V. Kamat, N.M. Dimitrijevic, and R.W. Fessenden, J. Phys. Chem. 1988, 92, 2324-9.
46. Electropolymerization of 9-vinylanthracene: Kinetic study using thin-layer spectroelectrochemistry. P.V. Kamat and S.K. Gupta, Polymer 1988, 29, 1329-34.
45. Photochemistry of 3,4,9,10-perylenetetracarboxylic dianhydride dyes. 3. Singlet and triplet excited-state properties of the bis(2,5-di-tert-butylphenyl)imide derivative. W.E. Ford and P.V. Kamat, J. Phys. Chem. 1987, 91, 6373-80.
44. Formation and corrosion processes of colloidal In2Se3. N.M. Dimitrijevic and P.V. Kamat, Langmuir 1987, 3, 1004-9.
43. Photochemistry on surfaces: Triplet-triplet energy transfer on colloidal TiO2 particles. P.V. Kamat and W.E. Ford, Chem. Phys. Lett. 1987, 135, 421-6.
42. Fluorescence emission as a probe to investigate electrochemical polymerization of 9-vinylanthracene. P.V. Kamat, Anal. Chem. 1987, 59, 1636-8.
41. Electron transfer reactions. Reactions of 1,2-dibenzoylalkenes and related substrates with potassium and oxygen. B. Pandey, M.P. Mahajan, R.K. Tikare, Ashok, K., Kamat, P.V., George, M.V. Can. J. Chem. -unpublished
40. Transient photobleaching of small CdSe colloids in acetonitrile. Anodic decomposition. N.M. Dimitrijevic and P.V. Kamat, J. Phys. Chem. 1987, 91, 2096-9.
39. Photoelectrochemistry in particulate systems. 6. Electron-transfer reactions of small CdS colloids in acetonitrile. P.V. Kamat, N.M. Dimitrijevic, and R.W. Fessenden, J. Phys. Chem. 1987, 91, 396-401.
38. Electron transfer reactions. Reaction of D2-oxazoline- 5-ones and related substrates with potassium. M. Muneer, R.K. Tikare, P.V. Kamat, and M.V. George, Can. J. Chem. 1987, 65, 1624-30.
37. Photoelectrochemistry in particulate systems. 5. Visible light-induced polymerization of 1-vinylpyrene in semiconductor suspensions. P.V. Kamat and R.V. Todesco, J. Polym. Sci., Part A, Polym. Chem. 1987, 25, 1035-40.
36. Electron transfer reactions. Reaction of nitrones with potassium. K. Ashok, P.M. Scaria, P.V. Kamat, and M.V. George, Can. J. Chem. 1987, 65, 2039-49.
35. Excited-state behavior of poly[dimethylsilylene-co-methyl(1-naphthyl)silylene]. R.V. Todesco and P.V. Kamat, Macromolecules 1986, 19, 196-200.
34. Photoelectrochemistry in particulate systems. 4. Photosensitization of a TiO2 semiconductor with a chlorophyll analogue. P.V. Kamat, J.P. Chauvet, and R.W. Fessenden, J. Phys. Chem. 1986, 90, 1389-94.
33. Photophysical and photochemical behavior of poly(1- vinylpyrene). Evidence for dual excimer fluorescence. R.V. Todesco, R.A. Basheer, and P.V. Kamat, Macromolecules 1986, 19, 2390-7.
32. Photosensitized charge injection into TiO2 particles as studied by microwave absorption. R.W. Fessenden and P.V. Kamat, Chem. Phys. Lett. 1986, 123, 233-8.
31. Electron transfer reactions. Reaction of furanones and bifurandiones with potassium and oxygen. B. Pandey, R.K. Tikare, M. Muneer, P.V. Kamat, and M.V. George, Chem. Ber. 1986, 119, 917-928.
1985-1981
30. Photoelectrochemistry in particulate systems. 3. Phototransformations in the colloidal titania-thiocyanate system. P.V. Kamat, Langmuir 1985, 1, 608-11.
29. Polymer-modified electrodes. Electrochemical and photoelectrochemical polymerization of 1-vinylpyrene. P.V. Kamat, R. Basheer, and M.A. Fox, Macromolecules 1985, 18, 1366-71.
28. Photoelectrochemistry in colloidal systems. Part 2. A photogalvanic cell based on TiO2 semiconductor colloid. P.V. Kamat, J. Chem. Soc., Faraday Trans. 1985, 1, 81, 509-18.
27. Photoelectrochemistry in colloidal systems: Interfacial electron transfer between colloidal TiO2 and thionine in acetonitrile. P.V. Kamat, J. Photochem. 1985, 28, 513-24.
26. Photoelectrochemical effect with poly(p-phenylene sulfide) films. P.V. Kamat and R.A. Basheer, Chem. Phys. Lett. 1984, 103, 503-6.
25. Electrochemistry and photoelectrochemistry of dye- incorporated clay-modified electrode. P.V. Kamat, J. Electroanal. Chem. Interfacial Electrochem. 1984, 163, 389-94.
24. Dye loaded polymer electrodes. III. Generation of photogalvanic effects at n-SnO2 electrodes coated with poly(4-vinyl pyridine) films containing rose bengal. P.V. Kamat and M.A. Fox, J. Electrochem. Soc. 1984, 131, 1032-7.
23. Triplet state properties of croconate dyes in homogeneous and polymer-containing solutions. P.V. Kamat and M.A. Fox, J. Photochem. 1984, 24, 285-92.
22. Dye-loaded polymer electrodes. 2. Photoelectrochemical sensitization of croconate violet in polymer films. P.V. Kamat, M.A. Fox, and A.J. Fatiadi, J. Am. Chem. Soc. 1984, 106, 1191-97.
21. Photophysics and photochemistry of xanthene dyes in polymer solutions and films. P.V. Kamat and M.A. Fox, J. Phys. Chem. 1984, 88, 2297-302.
20. Photosensitization of TiO2 colloids by erythrosin B in acetonitrile. P.V. Kamat and M.A. Fox, Chem. Phys. Lett. 1983, 102, 379-84.
19. Dye loaded polymer electrodes. I. The electrochemical behavior of n-SnO2 electrodes modified by adsorption of poly(4-vinyl pyridine) films containing an anionic dye. P.V. Kamat and M.A. Fox, J. Electroanal. Chem. 1983, 159, 49-62.
18. Time-resolved photoelectrochemistry. A laser-induced coulostatic flash study of n-TiO2 in acetonitrile. P.V. Kamat and M.A. Fox, J. Phys. Chem. 1983, 87, 59-63.
17. Chemically-modified electrodes in photoelectrochemical cells. M.A. Fox, J.R. Hohman, and P.V. Kamat, Can. J. Chem. 1983, 61, 888-93.
16. Temperature dependence of quenching rates and efficiencies of net forward and reverse electron transfer in the quenching of protonated triplet methylene blue by complexes of iron(II). P.V. Kamat and N.N. Lichtin, J. Phys. Chem. 1982, 86, 351-3.
15. Electron transfer in the quenching of protonated triplet thionine and methylene blue by ground state thionine. P.V. Kamat and N.N. Lichtin, J. Photochem. 1982, 18, 197-209.
14. Properties of the triplet state of N,N,N',N'- tetraethyloxonine. P.V. Kamat and N.N. Lichtin, Isr. J. Chem. 1982, 22, 113-6.
13. Enhanced fluorescence emission of croconate violet in ethanol containing poly(4-vinyl-pyridine). P.V. Kamat and M.A. Fox, Chem. Phys. Lett. 1982, 92, 595-9.
12. Kinetics of photobleaching recovery in the iron(II)- thionine system. P.V. Kamat, M.D. Karkhanavala, and P.N. Moorthy, J. Phys. Chem. 1981, 85, 810-3.
11. Electron transfer in the quenching of protonated triplet methylene blue by ground-state molecules of the dye. P.V. Kamat and N.N. Lichtin, J. Phys. Chem. 1981, 85, 814-8.
10. Photoinduced electron ejection from methylene blue in water and acetonitrile. P.V. Kamat and N.N. Lichtin, J. Phys. Chem. 1981, 85, 3864-8.
9. The pKa of diprotonated semimethylene blue, in 5% ethanol and 50% acetonitrile aqueous solutions. P.V. Kamat and N.N. Lichtin, Photochem. Photobiol. 1981, 33, 109-13.
8. Study of polarization in ferrous-thionine photogalvanic cell. P.V. Kamat, M.D. Karkhanavala, and P.N. Moorthy, Indian J. Chem., Sect. A 1981, 20, 718-720.
1980-1977
7. Investigation on the textural characteristics of supported nickel hydrogenation catalysts. G. Srinivasan, R.S. Murthy, K.M.V. Kumar, and P.V. Kamat, J. Chem. Tech. Biotechnol. 1980, 30, 217-224.
6. Temperature effects in photoelectrochemical cells. P.V. Kamat, M.D. Karkhanavala, and P.N. Moorthy, J. Appl. Phys. 1979, 50, 4228-4230.
5. Study of ferrous-thionine system. Part I. Photogalvanic effect in homogeneous type cells. P.V. Kamat, M.D. Karkhanavala, and P.N. Moorthy, Indian J. Chem., Sect. A 1979, 18, 206-9.
4. Study of ferrous-thionine system. Part II. Power output in homogeneous and heterogeneous type photogalvanic cells. P.V. Kamat, M.D. Karkhanavala, and P.N. Moorthy, Indian J. Chem. 1979, Sect. A, 18, 210-2.
3. Thermophotoelectrochemical cells for solar energy conversion. P.V. Kamat, M.D. Karkhanavala, and P.N. Moorthy, Sol. Energy 1978, 20, 171-173.
2. Enhancement of the power output of photogalvanic cells. P.V. Kamat, M.D. Karkhanavala, and P.N. Moorthy, Ind. J. Chem. 1977, 15A, 342-344.
1. Photochemical and Photoelectrochemical Routes for solar energy conversion. P.N. Moorthy, J.P. Mittal, M.D. Karkhanavala, A. Sapre, V., T. Mukherjee, S.K. Sarkar, and P.V. Kamat. In National Solar Energy Congress. 1976. Calcutta, India.
