News From the Kamat Lab
Come listen to Dr. Kamat and Rebecca Scheidt present our latest research at MRS Boston 2018!
Click here for MRS 2018 full Program / Exhibit guide.

Dr. Kamat will be speaking on Monday, November 26th at 10:15am (ET) in Hynes Convention Center, Level 3 Ballroom C. The talk is entitled "Charge Injection from Excited CsPbBr3 Nanocrystals into TiO2 in Perovskite and Its Role in the Degradation of Perovskite Layer in Visible Light". Come listen to the latest research from the Kamat Lab!
Rebecca Scheidt, third year graduate student in the lab, will be speaking on Thursday, November 29th at 9am (ET) in Hynes Convention Center, Level 3 Room Ballroom B. The talk is entitled "Suppression of Halide Ion Exchange in Cesium Lead Halide Perovskites with PbSO4-Oleate Capping". Come learn what exciting things the Kamat Lab is up to!
Interfacial Charge Transfer between Excited CsPbBr3 Nanocrystals and TiO2: Charge Injection versus Photodegradation
Click here to listen to Rebecca Scheidt - third year grad student and first author of this paper - talk about her project via a LiveSlides presentation!
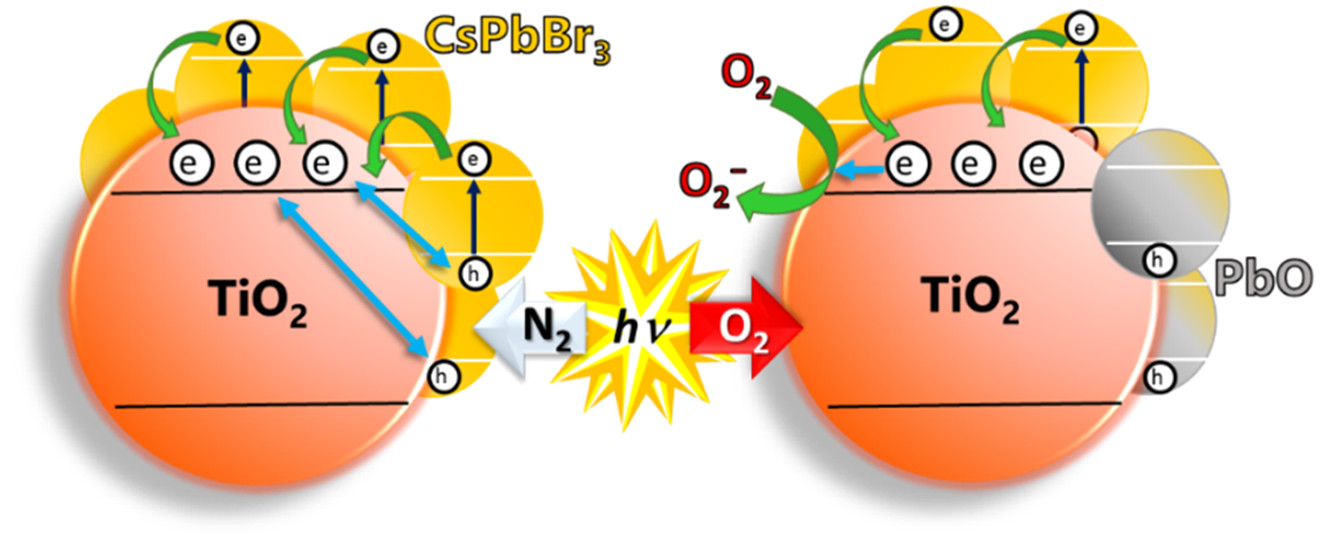
Abstract: Record-breaking efficiency achieved with quantum dot solar cells made of perovskite nanocrystals demands understanding of the excited-state interactions between perovskite nanocrystals and metal oxide electron transport layers. The interfacial electron transfer between excited CsPbBr3 perovskite nanocrystals and metal oxides (TiO2, SnO2, and ZnO) was elucidated using transient absorption spectroscopy and found to occur with a rate constant in the range of 2–4 × 1010 s–1. In an inert atmosphere, back electron transfer helps to maintain the stability of the perovskite nanocrystals. However, the presence of oxygen introduces instability as it scavenges away transferred electrons from the electron-transporting metal oxide, leaving behind holes to accumulate at CsPbBr3 nanocrystals, which in turn induce anodic corrosion. X-ray photoelectron spectroscopy measurements have enabled us to identify PbO as the major photodegraded product. The importance of the surrounding atmosphere and the supporting metal oxide in governing the stability of perovskite nanocrystals is discussed.
Transformation of Sintered CsPbBr3 Nanocrystals to Cubic CsPbI3 and Gradient CsPbBrxI3-x through Halide Exchange
Read the latest paper from the Kamat Lab!
Transformation of Sintered CsPbI3 Nanocrystals to Cubic CsPbI3 and Gradient CsPbBrxI3-x through Halide Exchange
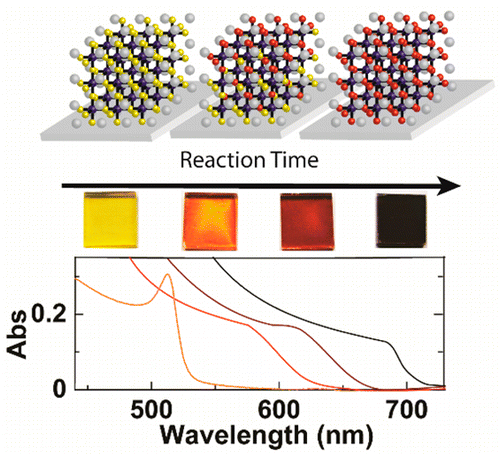
Abstract: All-inorganic cesium lead halide (CsPbX3, X = Br-, I-) perovskites could potentially provide comparable photovoltaic performance with enhanced stability compared to organic-inorganic lead halide species. However, small-bandgap cubic CsPbI3 has been difficult to study due to challenges forming CsPbI3 in the cubic phase. Here, a low-temperature procedure to form cubic CsPbI3 has been developed through a halide exchange reaction using films of sintered CsPbBr3 nanocrystals. The reaction was found to be strongly dependent upon temperature, featuring an Arrhenius relationship. Additionally, film thickness played a significant role in determining internal film structure at intermediate reaction times. Thin films (50 nm) showed only a small distribution of CsPbBrxI3-x species, while thicker films (350 nm) exhibited much broader distributions. Furthermore, internal film structure was ordered, featuring a compositional gradient within film. Transient absorption spectroscopy showed the influence of halide exchange on the excited state of the material. In thicker films, charge carriers were rapidly transferred to iodide-rich regions near the film surface within the first several picoseconds after excitation. This ultrafast vectorial charge-transfer process illustrates the potential of utilizing compositional gradients to direct charge flow in perovskite-based photovoltaics.
Tracking Iodide and Bromide Ion Segregation in Mixed Halide Lead Perovskites during Photoirradiation
Read the latest paper from the Kamat Lab!
Tracking Iodide and Bromide Ion Segregation in Mixed Halide Lead Perovskites during Photoirradiation
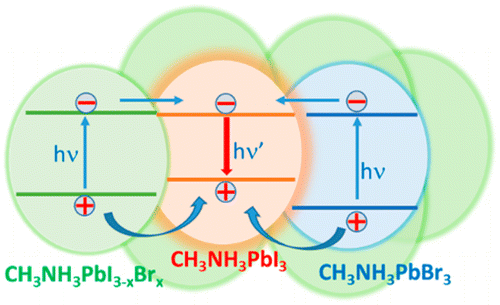
Abstract: Mixed halide lead perovskites (e.g., CH3NH3PbBrxI3-x) undergo phase segregation creating iodide-rich and bromide-rich domains when subjected to visible irradiation. This intriguing aspect of halide ion movement in mixed halide films is now being tracked through excited-state behavior using emission and transient absorption spectroscopy tools. These transient experiments have allowed us to establish the time scale with which such separation occurs under laser irradiation (405 nm, 25 mW/cm2 to 1.7 W/cm2) as well as dark recovery. While the phase separation occurs with a rate constant of 0.1-0.3 s-1, the recovery occurs over a time period of several minutes to an hour. The relative photoluminescence quantum yield observed for Br-rich regions (em. max 530 nm) is nearly 2 orders of magnitude lower than that of I-rich regions (em. max 760 nm) and arises from the fact that I-rich regions serve as sinks for photogenerated charge carriers. Understanding such cascading charge transfer to localized sites could further enable the design of gradient halide structures in mixed halide systems.
Intriguing Optoelectronic Properties of Metal Halide Perovskites
Read the latest paper from the Kamat Lab!
Intriguing Optoelectronic Properties of Metal Halide Perovskites
Abstract: A new chapter in the long and distinguished history of perovskites is being written with the breakthrough success of metal halide perovskites (MHPs) as solution-processed photovoltaic (PV) absorbers. The current surge in MHP research has largely arisen out of their rapid progress in PV devices; however, these materials are potentially suitable for a diverse array of optoelectronic applications. Like oxide perovskites, MHPs have ABX3 stoichiometry, where A and B are cations and X is a halide anion. Here, the underlying physical and photophysical properties of inorganic (A = inorganic) and hybrid organic-inorganic (A = organic) MHPs are reviewed with an eye toward their potential application in emerging optoelectronic technologies. Significant attention is given to the prototypical compound methylammonium lead iodide (CH3NH3PbI3) due to the preponderance of experimental and theoretical studies surrounding this material. We also discuss other salient MHP systems, including 2-dimensional compounds, where relevant. More specifically, this review is a critical account of the interrelation between MHP electronic structure, absorption, emission, carrier dynamics and transport, and other relevant photophysical processes that have propelled these materials to the forefront of modern optoelectronics research.
Kamat Lab Ready for Summer 2016!

With only 3 days until the official start of the Summer solstice, the Kamat Lab is ready for another productive season! Stay tuned for more news on the exciting research being conducted here in the Kamat Lab!
Congratulations to Kamat Lab Alumnus Dr. Ian Lightcap!

ND Energy has announced that Dr. Ian Lightcap, Research and Facilities Program Director, is the recipient of the Presidential Achievement Award for his outstanding efforts in the Materials Characterization Facility (MCF). Please join us in congratulating Ian! He will formally receive the award in June from ND President, Father John Jenkins.
Congratulations Radiation Lab Students!

On Monday, we had the pleasure of celebrating the Radiation Laboratory's 2016 graduates on their successful Ph.D. and Master's defenses, with some delicious cake! Pictured from left to right are: Xuequian Zhang, Ph.D., (Prof. Ptasinska's student), Yong-Siou Chen, Ph.D., (Prof. Kamat's student), Dr. Carmichael (Radiation Lab Director), Dr. Ptasinska, Dr. Kamat, Sebastian Snowberger, M.Sc., (Prof. Kamat's student), and Joseph Manser, Ph.D., (Prof. Kamat's student). Once again, congratulations!
CONGRATULATIONS Dr. Joseph Manser!

Graduate student Joseph Manser from the Department of Chemical and Biomolecular Engineering has successfully defended his Ph.D. thesis from the Kamat Lab entitled "Hybrid Lead Halide Perovskites for Light Energy Conversion: Excited State Properties and Photovoltaic Applications." Way to go Joe! This is the second successful defense from the Kamat Lab for the academic year 2015-2016.
Two Distinct Transitions in CuxInS2 Quantum Dots. Bandgap versus Sub-Bandgap Excitations in Copper-Deficient Structures
Read the latest paper from the Kamat Lab!
Two Distinct Transitions in CuxInS2 Quantum Dots. Bandgap versus Sub-Bandgap Excitations in Copper-Deficient Structures
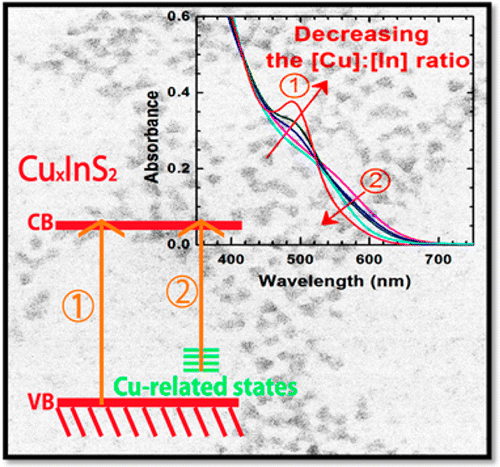
Abstract: Cu-deficient CuInS2 quantum dots (QDs) synthesized by varying the [Cu]:[In] ratio allow modulation of optical properties as well as identification of the radiative emission pathways. Absorption and emission spectral features showed a strong dependence on the [Cu]:[In] ratio of CuxInS2 QDs, indicating two independent optical transitions. These effects are pronounced in transient absorption spectra. The bleaching of band edge absorption and broad tail absorption bands in the subpicosecond-nanosecond time scale provide further evidence for the dual optical transitions. The recombination process as monitored by photoemission decay indicated the involvement of surface traps in addition to the bandgap and sub-bandgap transitions. Better understanding of the origin of the optical transitions and their influence on the photodynamics will enable utilization of ternary semiconductor quantum dots in display and photovoltaic devices.
CONGRATULATIONS Dr. Yong-Siou Chen!

Graduate student Yong-Siou Chen from the Department of Chemistry and Biochemistry has successfully defended his Ph.D. thesis from the Kamat Lab entitled "Organometal Halide Perovskites and Gold Nanoclusters for Solar Energy Conversion." Please join us in congratulating Dr. Chen!
TODAY: Lecture Series by World-Renowned Guests
TODAY ONLY! There will be a series of seminars hosted by the Kamat Lab group. Click the link below for more information!
How Lead Halide Complex Chemistry Dictates the Composition of Mixed Halide Perovskites
Read the latest paper from the Kamat Lab!
How Lead Halide Complex Chemistry Dictates the Composition of Mixed Halide Perovskites
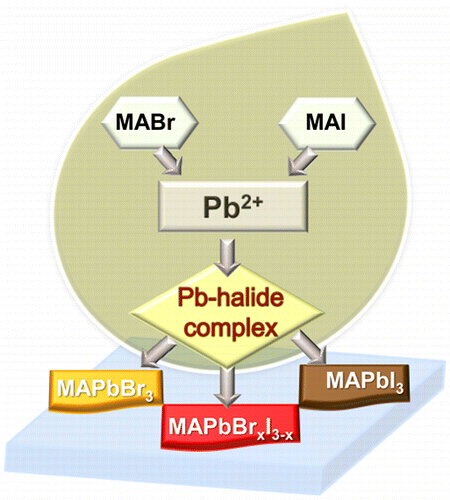
Abstract: Varying the halide ratio (e.g., Br-:I-) is a convenient approach to tune the bandgap of organic lead halide perovskites. The complexation between Pb2+ and halide ions is the primary step in dictating the overall composition, and optical properties of the annealed perovskite structure. The complexation between Pb2+ and Br- is nearly 7 times greater than the complexation between Pb2+ and I-), thus making Br- a dominant binding species in mixed halide systems. Emission and transient absorption measurements show a strong dependence of excited state behavior on the composition of halide ions employed in the precursor solution. When excess halide (X = Br- and I-) are present in the precursor solution (0.3 M PbX2 and 0.9 M CH3NH3X), the exclusive binding of Pb2+ with Br- results in the formation of CH3NH3PbBr3 perovskites as opposed to mixed halide perovskite.
Modulation of Cu2-xS Nanocrystal Plasmon Resonance through Reversible Photoinduced Electron Transfer
Read the latest paper from the Kamat Lab!
Modulation of Cu2-xS Nanocrystal Plasmon Resonance through Reversible Photoinduced Electron Transfer
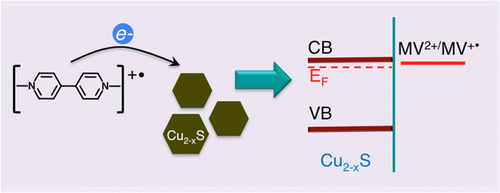
Abstract: Copper sulfide (Cu2-xS) nanocrystals with nonstoichiometric composition exhibit plasmon resonance in the near-infrared region. Compositional changes and varying electron density markedly affect the position and intensity of the plasmon resonance. We report a photochemically induced phenomenon of modulating the plasmon resonance in a controlled fashion. As photogenerated reduced methyl viologen radicals transfer electrons to Cu2-xS in inert solutions, we observe a decrease in localized surface plasmon resonance (LSPR) absorbance at 1160 nm. Upon exposure to air, the plasmon resonance band recovers as stored electrons are scavenged away by oxygen. This cycle of electron charge and discharge of Cu2-xS nanocrystals is reversible and can be repeated through photoirradiation in N2 saturated solution followed by exposure of the suspension to air. The spectroscopic studies that provide mechanistic insights into the reversible charging and discharging of plasmonic Cu2-xS are discussed.
Prof. Kamat named editor-in-chief of ACS Energy Letters

Prof. Prashant Kamat has been named the editor-in-chief of ACS Energy Letters, effective March 1, 2016. Kamat is currently the deputy editor of The Journal of Physical Chemistry Letters. ACS Energy Letters is a newly launched peer-reviewed journal by The American Chemical Society. Join us in congratulating Prof. Kamat!
Spatially Non-uniform Trap State Densities in Solution-Processed Hybrid Perovskite Thin Films
Read the latest paper from the Kamat Lab!
Spatially Non-uniform Trap State Densities in Solution-Processed Hybrid Perovskite Thin Films
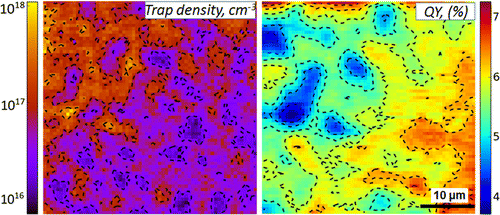
Abstract: The facile solution-processability of methylammonium lead halide (CH3NH3PbI3) perovskites has catalyzed the development of inexpensive, hybrid perovskite-based optoelectronics. It is apparent, though, that solution-processed CH3NH3PbI3 films possess local emission heterogeneities, stemming from electronic disorder in the material. Herein we investigate the spatially resolved emission properties of CH3NH3PbI3 thin films through detailed emission intensity versus excitation intensity measurements. These studies enable us to establish the existence of nonuniform trap density variations wherein regions of CH3NH3PbI3 films exhibit effective free carrier recombination while others exhibit emission dynamics strongly influenced by the presence of trap states. Such trap density variations lead to spatially varying emission quantum yields and correspondingly impact the performance of both methylammonium lead halide perovskite solar cells and other hybrid perovskite-based devices. Of additional note is that the observed spatial extent of the optical disorder extends over length scales greater than that of underlying crystalline domains, suggesting the existence of other factors, beyond grain boundary-related nonradiative recombination channels, which lead to significant intrafilm optical heterogeneities.
Dr. Catherine Murphy Delivers Inorganic Seminar at Notre Dame

Dr. Catherine J. Murphy is the Peter C. and Gretchen Miller Markunas Professor of Chemistry at The University of Illinois at Urbana-Champaign. She delivered the Inorganic Chemistry seminar today at Notre Dame titled "Gold Nanocrystals: Physics, Chemistry, Biology and Ecology." Pictured from left to right are Christian Talavera, Rabeka Alam, Dr. Catherine J. Murphy, and Yong-Siou Chen. We had the pleasure of having lunch with Prof. Murphy. Make sure to visit her website to learn more about her exciting research!
Evolution of Chemical Composition, Morphology, and Photovoltaic Efficiency of CH3NH3PbI3 Perovskite under Ambient Conditions
Read the latest paper from the Kamat Lab!
Evolution of Chemical Composition, Morphology, and Photovoltaic Efficiency of CH3NH3PbI3 Perovskite under Ambient Conditions
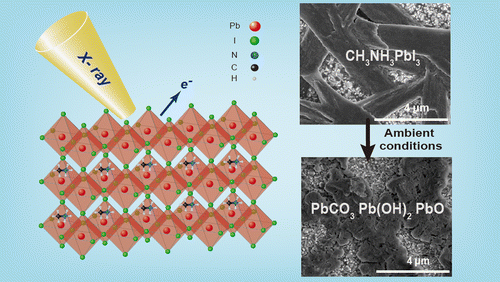
Abstract: The surface composition and morphology of CH3NH3PbI3 perovskite films stored for several days under ambient conditions were investigated by X-ray photoelectron spectroscopy, scanning electron microscopy, and X-ray diffraction techniques. Chemical analysis revealed the loss of CH3NH3+ and I- species from CH3NH3PbI3 and its subsequent decomposition into lead carbonate, lead hydroxide, and lead oxide. After long-term storage under ambient conditions, morphological analysis revealed the transformation of randomly distributed defects and cracks, initially present in the densely packed crystalline structure, into relatively small grains. In contrast to PbI2 powder, CH3NH3PbI3 exhibited a different degradation trend under ambient conditions. Therefore, we propose a plausible CH3NH3PbI3 decomposition pathway that explains the changes in the chemical composition of CH3NH3PbI3 under ambient conditions. In addition, films stored under such conditions were incorporated into photovoltaic cells, and their performances were examined. The chemical changes in the decomposed films were found to cause a significant decrease in the photovoltaic efficiency of CH3NH3PbI3.
Congratulations Jacob!

Fourth-year graduate student Jacob Hoffman has been selected to receive the 2016 Patrick and Jana Eilers Graduate Student Fellowship for Energy Related Research. This was a competitive year for the fellowship, so congratulations Jacob on this outstanding achievement!
Congratulations Dr. Prashant V. Kamat!

Thomson Reuters has named our very own Dr. Prashant V. Kamat among the top 1 percent of highly cited scholars. He was also named in previous years including the 2014 list. Congratulations Prashant on another successful year!
Kamat Lab 2015 Annual Report

Happy New Year! The 2015 Annual Report for the Kamat Lab has just been uploaded, highlighting our accomplishments in 2015. Thanks to all the graduate students, postdocs, undergrads, visiting scientists, and other collaborators for making this possible; and congratulations to everyone on their achievements and accomplishments! See the attached report for details, and here's hoping for an even better 2016!
Bill Gates Energy Innovation Paper references Kamat Lab!

While attending the international conference on climate change and energy, Bill Gates published a blog post detailing specifics on Mission Innovation and the Breakthrough Energy Coalition, two initiatives backed by Gates with the intent to invest in innovative ideas for renewable energy from the lab to the marketplace. Alongside the less formal post, he published a brief paper on Energy Innovation, which gave an overview of the current state of research and marketing in the field. The paper closed with three examples of "Promising Research", the last of which was the Kamat Lab's solar paint project! Follow the link below to read the blog post, which includes the Energy Innovation article.
LAB RATS WIN! LAB RATS WIN!

After a grueling offseason full of chemistry, publishing and conferences, the Rad Lab Lab Rats shed their nitrile gloves and safety glasses for softball gloves and shades last night for the first game of the annual intramural softball season. The Lab Rats took on the formidable and reigning softball champs the Cap'n Crunch Cereal Killers. We got straight to work in the first inning with Kevin blasting our first home run of the night deep into right field going up by two runs. With bases loaded and butts out of seats, Joe took to home plate and cranked out yet another home run in the first for a grand slam putting the Lab Rats up 6-0. Our defense ran the field and held the Cereal Killers to just one run after some fancy acrobatics by our outfielders Steve and Mark. After all that excitement in the first, we kept quiet in the second inning and regrouped while holding the Cereal Killers to what would be their last run of the game. Top of the third saw our very own, Korean grown lefty allstar Joon take to home plate with Sooraj on base. While he may have been unsure which side of home plate to stand on or how to hold the bat, that didn't stop him from hitting a grounder to the infield and setting Ken up for our third homerun of the night putting the Lab Rats up 9-2. After we scored 4 more runs and had some fancy glove work by Maj-Britt at third base, the Cereal Killers admitted defeat after 5 innings giving the Lab Rats their second victory in franchise history at 13-2. We're happy to report that everyone in our batting line-up connected, even Tessa from Ireland confusing softball for Rounders, striking fear in the heart of all of our opponents. Come on out to the West Quad fields behind Hammes Bookstore next Tuesday at 6:30 as the Lab Rats take on Team EM!
Multifaceted Excited State of CH3NH3PbI3. Charge Separation, Recombination, and Trapping
Read the latest paper from the Kamat Lab!
Multifaceted Excited State of CH3NH3PbI3. Charge Separation, Recombination, and Trapping
Abstract: A need to understand the excited-state behavior of organic–inorganic hybrid perovskites, such as CH3NH3PbI3, has arisen due to the rapid development of perovskite solar cells. The photoinduced processes leading to the efficient charge separation observed in these materials remain somewhat elusive. This Perspective presents an overview of the initial attempts to characterize the excited-state and charge recombination dynamics in the prototypical material CH3NH3PbI3. While much has been accomplished in designing high-efficiency solar cells, the multifaceted nature of the CH3NH3PbI3 excited state offers ample challenges for the photovoltaic community to better comprehend. Building on this foundation may enable us to tackle the stability concerns that have shadowed the rise of perovskite solar cells. Furthermore, a better understanding of the excited-state properties can provide insight into the specific properties that have thrust this material to the forefront of photovoltaic research.
Synergistic Effects in the Coupling of Plasmon Resonance of Metal Nanoparticles with Excited Gold Clusters
Read the latest paper from the Kamat Lab!
Synergistic Effects in the Coupling of Plasmon Resonance of Metal Nanoparticles with Excited Gold Clusters
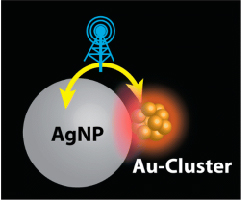
Abstract: When molecules or clusters are within the proximity of metal particles, their electronic transitions can be drastically enhanced. We have now probed the off-resonance excitation of molecule-like, glutathione-capped gold clusters (Au-GSH) in the close proximity of larger (plasmonic) Au and Ag nanoparticles. The excited state absorption spectrum of Au-GSH* is obtained with monophotonic excitation. The characteristic absorption of Au-GSH* allows us to probe the influence of excited plasmonic nanoparticles coupled with the clusters. Although infrared (775 nm) lasers pulses do not produce Au-GSH*, the excited states of these clusters are formed when coupled with metal (Au, Ag) nanoparticles. Interestingly, the coupled excitation of Au-GSH/AgNP with 775 nm laser pulses also results in an enhanced field effect, as seen from increased plasmon response of the metal nanoparticles. Transient absorption measurements confirm the synergy between these two inherently different nanomaterials, causing them to display greater excitation features. Better understanding of metal cluster–metal nanoparticle interactions will have important implications in designing light harvesting systems, and optoelectronic devices.
Congratulations to Dr. Jeffrey Christians and Dr. Douglas Hines!

Congratulations to graduate students Jeffrey Christians and Douglas Hines, who completed their Ph.D.s in Chemical and Biomolecular Engineering and Chemistry, respectively!
Jeff successfully defended his thesis entitled:
Mesostructured Thin Film Solar Cells: Examining Hole Transfer Mechanisms and Device Stability,
and was awarded the Eli J. and Helen Shaheen Graduate School Award in the Division of Engineering.
Doug successfully defended his thesis entitled:
Excited State Reactions at the Quantum Dot Surface,
and was awarded the Eli J. and Helen Shaeen Graduate School Award in the Division of Science.
Congratulations for completion of your Ph.D.s and having your excellent work recognized by the Graduate School!
Jeff Christians - National Renewable Energy Laboratory Postdoc

Join us in congratulating graduate student Jeffrey Christians who will be joining the National Renewable Energy Laboratory in Golden, CO (a suburb of Denver) as a postdoc with Dr. Joseph Luther! Jeff will be starting at NREL in May and will be working with Dr. Luther and others there to further develop and understand perovskite solar cells.
Humidity Effects on CH3NH3PbI3
Read the latest paper from the Kamat Lab!
Transformation of the Excited State and Photovoltaic Efficiency of CH3NH3PbI3 Perovskite upon Controlled Exposure to Humidified Air
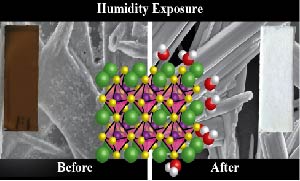
Abstract: Humidity has been an important factor, in both negative and positive ways, in the development of perovskite solar cells, and will prove critical in the push to commercialize this exciting new photovoltaic technology. The interaction between CH3NH3PbI3 and H2O vapor is investigated by characterizing the ground state and excited state optical absorption properties, and probing morphology and crystal structure. These systematic undertakings elucidate the complex interaction inherent in this system, demonstrating that H2O exposure does not simply only CH3NH3PbI3 to revert to PbI2. It is shown that, in the dark, H2O is able to complex with the perovskite, forming a hydrate product similar to (CH3NH3)4PbI6•2H2O. This causes a decrease in absorption across the visible region of the spectrum and a distinct change in the crystal structure of the material. Femtosecond transient absorption spectroscopic measurements show the effect that humidity has on the ultrafast excited state dynamics of CH3NH3PbI3. More importantly, the deleterious effects of humidity on complete solar cells, specifically on photovoltaic efficiency and stability, are explored in light of these spectroscopic understandings.
Water Splitting with CH3NH3PbI3/BiVO4
Read the latest paper from the Kamat Lab!
All Solution-Processed Lead Halide Perovskite-BiVO4 Tandem Assembly for Photolytic Solar Fuels Production
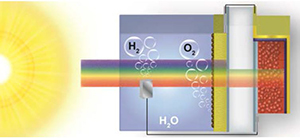
Abstract: The quest for economic, large scale hydrogen production has motivated the search for new materials and device designs capable of splitting water using only energy from the sun. Here we introduce an all solution-processed tandem water splitting assembly composed of a BiVO4 photoanode and a single-junction CH3NH3PbI3 hybrid perovskite solar cell. This unique configuration allows efficient solar photon management, with the metal oxide photoanode selectively harvesting high energy visible photons and the underlying perovskite solar cell capturing lower energy visible-near IR wavelengths in a single-pass excitation. Operating without external bias under standard AM 1.5G illumination, the photoanode-photovoltaic architecture, in conjunction with an earth-abundant cobalt phosphate catalyst, exhibits a solar-to-hydrogen conversion efficiency of 2.5% at neutral pH. The design of low-cost tandem water splitting assemblies employing single-junction hybrid perovskite materials establishes a potentially promising new frontier for solar water splitting research.
Chemistry of Materials - Cover Article
 Congratulations to graduate student Danilo Jara on the cover art for the journal Chemistry of Materials! The cover art appeared in this month's issue of Chemistry of Materials to go along with Danilo, Joon, Kevin, and Prashant's article on the size effect of CuInS2 entitled, Size-Dependent Photovoltaic Performance of CuInS2 Quantum Dots Sensitized Solar Cells.
Congratulations to graduate student Danilo Jara on the cover art for the journal Chemistry of Materials! The cover art appeared in this month's issue of Chemistry of Materials to go along with Danilo, Joon, Kevin, and Prashant's article on the size effect of CuInS2 entitled, Size-Dependent Photovoltaic Performance of CuInS2 Quantum Dots Sensitized Solar Cells.
About the Image: CuInS2 quantum dots with pyramidal shape display size-dependent photovoltaic performance. The origin of the photocurrent was found to arise from defect states, and an optimal size was identified based on charge stabilization.
Predicting ket at the CdSe-Linker-TiO2 Interface
Read the latest paper from the Kamat Lab!
Predicting the Rate Constant of Electron Tunneling Reactions at the CdSe-Linker-TiO2 Interface
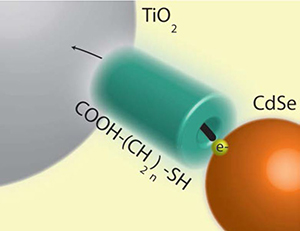
Abstract: Current interest in quantum dot solar cells (QDSCs) motivates an understanding of the electron transfer dynamics at the quantum dot (QD) – metal oxide (MO) interface. Employing transient absorption spectroscopy, we have monitored the electron transfer rate (ket) at this interface as a function of the bridge molecules that link QDs to TiO2. Using mercaptoacetic acid (MAA), 3-mercaptopropionic acid (3-MPA), 8-mercaptooctanoic acid (8-MOA) and 16-mercaptohexadecanoic acid (16-MHA) we observe an exponential attenuation of ket with increasing linker length, which has been attributed to the tunneling of the electron through the insulating linker molecule. We model the electron transfer reaction using both rectangular and trapezoidal barrier models that have been discussed in the literature. The one electron reduction potential (equivalent to the lowest unoccupied molecular orbital or LUMO) of each molecule as determined by cyclic voltammetry (CV) was used to estimate the effective barrier height presented by MAA, 3-MPA, 8-MOA and16-MHA at the CdSe-TiO2 interface. The electron transfer rate (ket) calculated for each CdSe-TiO2 interface using both models showed the results in agreement with the experimentally determined trend. This demonstrates that electron transfer between CdSe and TiO2 can be viewed as electron tunneling through a layer of organic linking molecules and provides a useful method for predicting electron transfer rate constants.
Boosting VOC using Gold Clusters
Read the latest paper from the Kamat Lab!
Boosting the Photovoltage of Dye-Sensitized Solar Cells with Thiolated Gold Nanoclusters
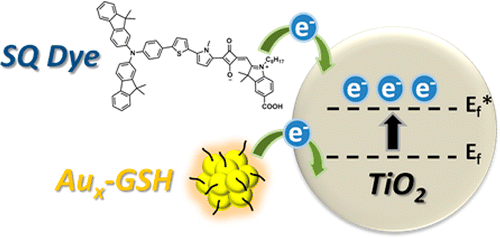
Abstract: Glutathione-capped gold nanoclusters (Aux-GSH NCs) are anchored along with a sensitizing squaraine dye on a TiO2 surface to evaluate the cosensitizing role of Aux-GSH NCs in dye-sensitized solar cells (DSSCs). Photoelectrochemical measurements show an increase in the photoconversion efficiency of DSSCs when both sensitizers are present. The observed photoelectrochemical improvements in cosensitized DSSCs are more than additive effects as evident from the increase in photovoltage (ΔV as high as 0.24 V) when Aux-GSH NCs are present. Electron equilibration and accumulation within gold nanoclusters increase the quasi-Fermi level of TiO2 closer to the conduction band and thus decrease the photovoltage penalty. A similar beneficial role of gold nanoclusters toward boosting the Voc and enhancing the efficiency of Ru(II) polypyridyl complex-sensitized solar cells is also discussed.
Elkhart Memorial High School

We recently assisted 12 chemistry students from Elkhart Memorial High School, and their teacher Brenda Mueller, in a lab experiment on Dye-Sensitized Solar Cells. Brenda had participated in the summer RET program at Notre Dame where she learned about solar cells, and then brought that knowhow back to her students at EMHS. Jeff, Joe, and Danilo taught them about solar cells, tested devices that they had made in their lab, and gave them a tour of our laboratories - we even got to eat some Papa John's! Thanks to all the students for making it a fun visit!
KamatLab 2014 Annual Report

2014 has been another very successful year in the Kamat Lab thanks to all of the hardworking graduate students, postdocs, visiting scientists, undergraduates, and collaborators! Two members of the group received PhDs, Sachi Krishnamurthy in Chem. and James Radich in CBE, and we welcomed two new graduate students to the group, Christian Telavera in Chem. and Sebastian Snowberger in CBE. In addition, we welcomed postdoc Chris Tuinenga to the group. As a group, we were able to publish 28 research articles - setting a new record for the group! - in many different high-impact journals since our last Annual Report. Many of our graduate students, undergraduates, and postdocs were also recipients of numerous fellowships and awards, and presented their research at international conferences. It has been an exciting year of research and we look forward to a productive and fun 2015! Best wishes for the New Year!
Yong-Siou Chen - Eilers Fellowship

Congratulations to Yong-Siou Chen who was recently awarded the 2015 Patrick and Jana Eilers Graduate Student Fellowship for Energy Related Research awarded by ND Energy. Yong-Siou received this prestigious award in order to continue his cutting-edge research on perovskite solar cells. He is currently a 4th year graduate student in the department of chemistry and the author of 5 publications as a member of the Kamat Lab, including 2 first author papers in JACS. Join us in congratulating Yong-Siou on this well-deserved recognition of his outstanding work!
Size-Dependent Photovoltaic Performance of CuInS2
Read the latest paper from the Kamat Lab!
Size-Dependent Photovoltaic Performance of CuInS2 Quantum Dots Sensitized Solar Cells
Abstract: The optical and electronic properties of quantum dots (QDs) which are drastically affected by their size have major impact on their performance in devices like solar cells. We now report the size dependent solar cell performance for CuInS2 QDs capped with 1-dodecanethiol. Pyramidal shaped CuInS2 QDs with diameter between 2.9 nm and 5.3 nm have been synthesized and assembled on mesoscopic TiO2 films by electrophoretic deposition. Time resolved emission and transient absorption spectroscopy measurements have ascertained the role of internal and surface defects in determining the solar cell performance. An increase in power conversion efficiency (PCE) was observed with increasing size of QDs, with maximum values of 2.14 and 2.51% for 3.9 and 4.3 nm size particles, respectively. The drop in PCE observed for larger QDs (5.3 nm) is attributed to decreased charge separation following bandgap excitation. Since the origin of photocurrent generation in CuInS2 QDSC arises from the defect dominated charge carriers it offers the opportunity to further improve the efficiency by controlling these defect concentrations.
Dual Nature of CH3NH3PbI3 Excited State
Read the latest paper from the Kamat Lab!
Dual Nature of the Excited State in Organic-Inorganic Lead Halide Perovskites
Abstract: The rapid increase in efficiency of methylammonium lead halide perovskite solar cells necessitates further investigation into the nature of perovskite absorption features and optical properties. Films obtained from the deposition of solutions containing lead halides and the CH3NH3+ organic cation is known to yield the CH3NH3PbI3 perovskite structure upon annealing. In examining the precursor solution used in the processing of CH3NH3PbI3 solar cells, we find that Pb2+ readily forms plumbate complexes in the presence of excess iodide ions and exhibits characteristic absorption bands at 370 (PbI3-) and 425 nm (PbI42-). Through comparative spectral analysis of the absorption features of charge transfer complexes in the solution phase and the final solid-state perovskite films, we are able to fully classify the absorption features in the excited state of CH3NH3PbI3 across the transient absorption spectrum recorded following laser pulse excitation. In particular, we attribute the broad photoinduced absorption to a charge-transfer excited state, and show correlation between the photoinduced absorption and 480 nm bleach signals. These observations lead us to propose a band structure composed of two distinct transitions that is consistent with the various spectral features and kinetic behavior of the CH3NH3PbI3 excited state. Characterization of this unique dual excited state nature provides further insight into the optoelectronic behavior of hybrid lead halide perovskite films and thus aids in elucidating their exceptional photovoltaic properties.
Kamat Lab Papers Make Most Read Lists
The past year has been an exciting and productive time in the Kamat Lab! Below are 7 articles that currently feature among the Most Read Articles in their respective journals over the past 12 months. Congratulations to all of the various authors who have contributed to this exciting work!
467. Band Filling with Free Charge Carriers in Organometal Halide Perovskites Manser, J. S.; Kamat, P. V. Nat. Photon. 2014, 8, 737–743.
458. Quantum Dot Solar Cells. Hole Transfer as a Limiting Factor in Boosting Photoconversion Efficiency Kamat, P. V.; Christians, J. A.; Radich, J. G. Langmuir 30 (20), 5716-5725 (Feature Article).
454. Recent Advances in Quantum Dot Surface Chemistry Hines, D. A.; Kamat, P. V. ACS Appl. Mater. Interfaces 2014, 6 (5), 3041–3057.
449. An Inorganic Hole Conductor for Organo-Lead Halide Perovskite Solar Cells. Improved Hole Conductivity with Copper Iodide Christians, J. A.; Fung, R. C. A.; Kamat, P. V. J. Am. Chem. Soc. 2014, 136 (2), 758-764.
438. Quantum Dot Solar Cells. The Next Big Thing in Photovoltaics. Kamat, P. V. J. Phys. Chem. Lett. 2013, 4, 908–918.
374. Quantum Dot Solar Cells. Semiconductor Nanocrystals as Light Harvestors - Centennial Feature Article Kamat, P. V. J. Phys. Chem. C 2008, 112, 18737-18753 . NDRL 4770
353. Meeting the Clean Energy Demand: Nanostructure Architectures for Solar Energy Conversion. Kamat, P. V. J. Phys. Chem. C 2007, 111, 2834-2860. (Feature Article in February 22 2007 issue) NDRL 4697
Editorial: Mastering the Art of Scientific Publication
"As new researchers generate their first results, they face the challenge of mastering the art of scientific publication in order to present their results and to draw attention to their new scientific findings. Whether or not we want to describe science in such terms, scientific publishing is competitive in nature, and thus younger scientists must vie with their more experienced peers for recognition. While the electronic age has made the publication process easier and quicker, optimizing the structure of a scientific paper requires a certain degree of skill and proficiency.(1) ACS Publications has been actively engaged in disseminating the basics of publication through Publication 101 videos and editorials and, in continuation of this spirit, we have assembled this virtual issue (http://pubs.acs.org/page/vi/art_of_scientific_publication.html). This issue draws together, in one place, these editorials that summarize the key steps involved in writing an effective paper, journal submission, review processes, and postpublication efforts. The twenty editorials assembled for this virtual issue provide further details on each of these topics. These topics may also be useful as part of the curriculum for the training of students and young researchers in any academic department."
Sachi to Los Alamos

Join us in congratulating Dr. Sachidananda Krishnamurthy on a great postdoc position at both UT Dallas and Los Alamos National Laboratory! Sachi will be working under the mentorship of Dr. Jennifer Hollingsworth at Los Alamos and Dr. Anton Malko and UT Dallas. He will be working on hybrid near IR QDSCs. Congratulations Sachi!
Editorail: What's in a Name?
"What is the first thing that draws the attention of any reader to look into a scientific paper? Naturally, it is the title. The title is something that stays around for the life of a paper, similar to an inherited name. Given the volume of papers being published in any given discipline in recent years, one can easily miss reading a paper if the article title listed in a journal's table of contents or in database search results fails to draw the attention of a potential reader. Is it not worth an author's time to come up with an effective and attractive title?"
Editorail: Reporting on Heterogeneous Photocatalysis
This editorial written for ACS Applied Materials and Interfaces looks into the best practices for reporting on hetergeneous photocatalysis. It includes tips for making measurements as well as proper reporting of values and things to watch out for in the literature.
Best Practices for Reporting on Heterogeneous Photocatalysis
Heterogeneous photocatalysis is of broad interest in materials chemistry and materials science, particularly with the rapid growth of research attention being directed toward energy-related applications, pollution mitigation, and other related areas of environmental impact.(1) A literature survey reveals more than 9000 papers with the word photocatalyst or photocatalysis in the title published during the last ten years (Source: Web of Science, July 3, 2014), with the number of papers published each year increasing significantly since 2005. The materials and physical chemistry journals of the American Chemical Society receive a significant number of papers in the area of photocatalysis.
Catalytic Reduction of CO2 with TiO2
Read the latest paper from the Kamat Lab!
The Origin of Catalytic Effect in the Reduction of CO2 at Nanostructured TiO2 Films
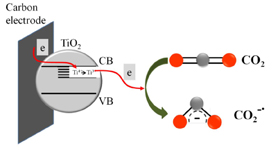
Abstract: Electrocatalytic activity of mesoscopic TiO2 films towards the reduction of CO2 is probed by depositing a nanostructured film on a glassy carbon electrode. The one-electron reduction of CO2 in acetonitrile seen at an onset potential of -1.1 V (vs. NHE) is ~0.5 V lower than the one observed with a glassy carbon electrode. The electrocatalytic role of TiO2 is elucidated through spectroelectrochemistry and product analysis. Ti3+ species formed when TiO2 film is subjected to negative potentials have been identified as active reduction sites. Binding of CO2 to catalytically active Ti3+ followed by the electron transfer facilitates the initial one-electron reduction process. Methanol was the primary product when the reduction was carried out in wet acetonitrile.
PhysOrg: Band Filling in Perovskite
PhysOrg: A new paper by University of Notre Dame researchers describes their investigations of the fundamental optical properties of a new class of semiconducting materials known as organic-inorganic "hybrid" perovskites.
The research was conducted at the Notre Dame Radiation Laboratory by Joseph Manser, a doctoral student in chemical and biomolecular engineering, under the direction of Prashant Kamat, Rev. John A. Zahm Professor of Science. The findings appear in a paper in the August 10 edition of the journal Nature Photonics.
Article continued on PhysOrg
C&EN: Graphene Surprises By Decomposing
C&EN: Graphene's hallmark chemical stability has made this ultrathin carbon network an ideal support material in catalysis and energy studies. But that inertness is now being called into question by an investigation showing that the material can decompose when used in common applications (Chem. Mater. 2014, DOI: 10.1021/cm5026552).
Water-dispersible forms of graphene are easy to make and easy to handle via simple wet-chemistry methods. Large numbers of researchers use the materials to support nanoparticle catalysts for use in environmental remediation, solar cells, and fuel cells.
Article continued on C&EN
Band Filling in Perovskite
Read the latest paper from the Kamat Lab!
Band filling with free charge carriers in organometal halide perovskites
Abstract: The unique and promising properties of semiconducting organometal halide perovskites have brought these materials to the forefront of solar energy research. Here, we present new insights into the excited-state properties of CH3>NH3PbI3 thin films through femtosecond transient absorption spectroscopy measurements. The photoinduced bleach recovery at 760 nm reveals that band-edge recombination follows second-order kinetics, indicating that the dominant relaxation pathway is via recombination of free electrons and holes. Additionally, charge accumulation in the perovskite films leads to an increase in the intrinsic bandgap that follows the Burstein–Moss band filling model. Both the recombination mechanism and the band-edge shift are studied as a function of the photogenerated carrier density and serve to elucidate the behaviour of charge carriers in hybrid perovskites. These results offer insights into the intrinsic photophysics of semiconducting organometal halide perovskites with direct implications for photovoltaic and optoelectronic applications.
Is Graphene Stable for Photocatalysis?
Read the latest paper from the Kamat Lab!
Is Graphene a Stable Platform for Photocatalysis? Mineralization of Reduced Graphene Oxide with UV-Irradiated TiO2 Nanoparticles
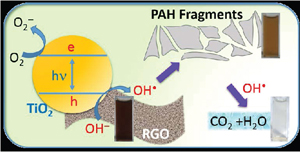
Abstract: The recent thrust in utilizing reduced graphene oxide (RGO) as a support for nanostructured catalyst particles has led to the claims of improved efficiency in solar cells, fuel cells, and photocatalytic degradation of pollutants. Specifically, the robust TiO2 system is often coupled with RGO to improve charge separation and facilitate redox reactions. Here we probe the stability of RGO in the presence of UV-excited TiO2 in aqueous media and establish its reactivity towards OH• radicals, a primary oxidant generated at the TiO2 surface. By probing changes in absorption, morphology and total organic carbon content (TOC) we conclusively demonstrate the vulnerability of RGO towards OH• attack and raise the concern of its use in many applications where OH• are likely to be formed. On the other hand, the OH• radical-mediated mineralization could also enable new approaches in tackling environmental remediation of nanocarbons such as RGO, carbon nanotubes, and fullerenes.
Size Dependent Excited State of Au Clusters
Read the latest paper from the Kamat Lab!
Size Dependent Excited State Behavior of Glutathione Capped Gold Clusters and Their Light Harvesting Capacity
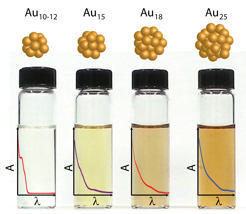
Abstract: Glutathione protected gold clusters exhibit size dependent excited state and electron transfer properties. Larger size clusters (e.g., Au25GSH18) with core-metal atoms display rapid (<1 ps) as well as slower relaxation (~200 ns) while homoleptic clusters (e.g., Au10-12GSH10-12) exhibit only slower relaxation. These decay components have been identified as metal-metal transition and ligand-to-metal charge transfer respectively. The short lifetime relaxation component becomes less dominant as the size of the gold cluster decreases. The long-lived excited state and ability to participate in electron transfer are integral for these clusters to serve as light harvesting antennae. A strong correlation between the ligand-to-metal charge-transfer excited state lifetime and photocatalytic activity was evidenced from the electron transfer to methyl viologen. The photoactivity of these metal clusters show increasing photocatalytic reduction yield (0.05 - 0.14) with decreasing cluster size, Au25 < Au18 < Au15 < Au10-12. Gold clusters, Au18GSH14, were found to have the highest potential as a photosensitizer based on the quantum yield of electron transfer and good visible light absorption properties.
Förster versus Dexter
Read the latest paper from the Kamat Lab!
Size Dependent Energy Transfer Pathways in CdSe Quantum Dot-Squaraine Light Harvesting Assemblies: Förster versus Dexter
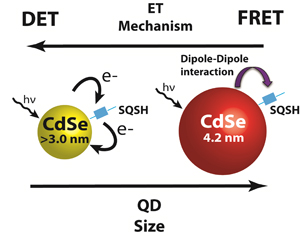
Abstract: Energy transfer coupled with electron transfer is a convenient approach to mimic photosynthesis in light energy conversion. Better understanding of mechanistic details of energy transfer processes is important to enhance the performance of dye or quantum dot sensitized solar cells. Energy transfer through both long range dipole based Förster Resonance Energy Transfer (FRET), and short range Dexter Energy Transfer (DET) mechanisms have been identified to occur between CdSe quantum dots (QDs) linked to a red-infrared absorbing squaraine dye through a short thiol functional group (SQSH). Solutions of SQSH linked to CdSe were investigated through steady-state and time resolved spectroscopy experiments to explore both mechanisms. Photoluminescence studies revealed that smaller QDs had higher energy transfer efficiencies than predicted by FRET, and femtosecond transient absorption experiments revealed faster energy transfer rates in smaller donor QD sizes. These findings supported A DET process dominating at small donor sizes. The presence of both processes illustrates multiple strategies for utilizing energy transfer in light harvesting assemblies and the required considerations in device design to maximize energy transfer gains through either mechanism.
Why Did You Accept My Paper?
This editorial written by the J. Phys. Chem. Lett. editors appears in today's issue. It highlights manuscript attributes contributing to favorable editorial decisions.
Why Did You Accept My Paper?
This is one question that we never hear from our authors. Authors whose papers get accepted by the editor cherish their publication success, whereas authors whose papers were not accepted may in many instances put the blame squarely on the review process itself. From the author's perspective, the paper he/she just submitted consists of the best scientific results from his/her laboratory and hence deserves publication regardless of the quality of presentation. Editors and reviewers evaluate the papers from the journal reader's viewpoint. Essential factors in drawing a reader's attention to a particular paper include a broad perspective, a good fit with the journal scope, and clear presentation of new scientific findings. Editors and reviewers also check to see whether the paper meets the journal's submission criteria. Those authors who take these aspects into consideration while composing their manuscripts see a significantly higher success rate of acceptance.
They Said it Couldn't be Done

Sometimes softball can be a metaphor for life. You can't let the fact that half your team doesn't know the difference between left field and right field stop you, you just have to get back up and keep swinging.
The doubters were proven wrong, the impossible was accomplished, the Lab Rats won a softball game! Some would point out that it took into our third season to get our first win, but I would point out that we actually had a win two years ago when the other team forfeited, so the thrill of victory is not totally unfamiliar territory. While our offense kept us competitive in our first game, it was our stifling defense that helped us prevail to our first win of the season ever. We held the MBA team to just 1 run in a game that stretched the full 7 innings. The tone was set in the first inning when Doug unleashed 100% power and hit a huge home run that scored two and put us up for good. The only hiccup was when Jay thought he could be like Doug and ended up getting out after being thrown into a pickle between third and home. Nevertheless, the final score of 6-1 smelled of glory and tasted of victory! If we win our next game we make the playoffs!
Opening Day at the Ballpark
The annual intramural softball season started up this week after the first game was rained out last week, and the Rad Lab Lab Rats walked onto the field to face the AME Bombers with fresh legs after taking the season off last year. Stepping up to the plate, we shook off the rust and jumped to an early 6-0 lead with some great (for us at least) hitting. Even Danilo, who was unsure whether the batter or pitcher was supposed to run after a hit, had a nice double! Then we took the field. Our defense is porous at best and a sieve at worst, and while I wouldn't say this was our worst outing, we quickly let the Bombers back into the game to tie it 6-6 after one. Danilo's play at second base to end the second inning was probably the highlight of the game. With two outs a hard grounder was hit just right of second base. Danilo ran over, reached out his glove and snagged the ball as it went past, and then, carried by his momentum, spun in a full circle before tossing the ball to Joe at second and getting the force out. Ultimately though, fatigue set in and the Lab Rats were not able to hold off the steady attack of the Bombers, eventually falling 16-11 in four innings. It was tough to start off the year with a loss but we will be back on the field next week hungry for our first win of the year!
Unfortunatly we forgot the camera today so there are no pictures but hopefully we will have some from our next game.
Editorial on Publication Figures
Read this editorial, entitled "Graphical Excellence," from Prashant, Journal of Physical Chemistry Letters Senior Editor Greg Hartland, and Editor-in-Chief George Schatz on the importance of high quality figures in conveying scientific information.
"It is the experience of every author that figures and illustrations in scientific papers are the gateway for effective communication of research findings to the scientific world. While the title and abstract of the paper draw the attention of any avid reader, the essence of the paper is captured by the figures and schemes. Well-drawn, scientifically correct figures make the first impression of the scientific findings. Yet, time and time again, we see poorly presented results, inaccuracy in plotted data, improperly defined axes, meaningless significant digits in axis scales, wrong or missing units, and undefined symbols or traces. Such inaccuracies can lead to reject recommendations from the editors and reviewers. We wonder why researchers who are passionate about communicating their findings, many times take little interest in effective presentation of their data."
Prashant Kamat - 2014 Highly Cited Researcher

Thomson Reuters compiled a list of approximately 3200 Highly Cited Researchers from around the world across 21 fields of the sciences and social sciences. Researchers were selected from each discipline who had published the most papers between 2001-2012 which ranked in the top 1% in citations relative to other papers published in the same field and in the same year. By this metric, three Notre Dame scientists made the list of Highly Cited Researchers, including Joan Brennecke (Chemistry), Bertrand Hochwald (Computer Science), and Prashant Kamat (Chemistry). Congratulations to all of the well deserving members of the 2014 Highly Cited Researchers list!
Kamat Lab: Summer 2014

With school offically over and summer beginning in South Bend, the Kamat Lab is starting to take on its summer form. New students and scientists will be joining us this summer, and several people will be leaving. This summer we are saying goodbye to recent Ph. D. graduates Sachidananda Krishnamurthy and James Radich, as well as visiting scientist Dr. Santosh Haram. However, we are also welcoming Ivan Grigioni, Barry Reid, Pierre Miranda, Mark Wilson, and Abby Swint who will be working in the lab over the summer. We look forward to an enjoyable and productive summer!
