Batteries
Background
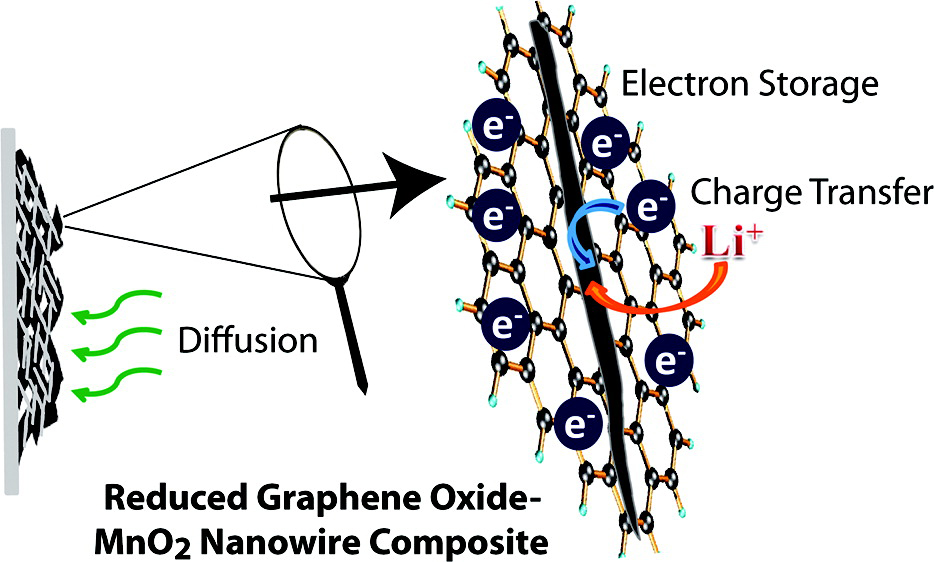
Figure. MnO2/RGO composites show improved Li-ion battery performance. The reason for the improvement was attributed to better electrode kinetics, more rapid diffusion of Li+ to intercalation sites, and a greater capacitance effect during discharge.
One of the key components that must be combined with an energy source, such as photovoltaics, that relies on sunlight is an affordable, robust energy storage method. This energy storage can be done in a variety of ways, but one of the most promising technologies is batteries. Lithium-ion batteries are of particular interest in the search for high capacity, light, batteries because of lithium's low molecular weight (6.9 g/mol) and high discarge voltage (typically 3+ volts). This gives Li-ion batteries the highest specific energy (energy per weight) of any type of battery. In Li-ion batteries, upon discharge, Li+ ions move from the negative to the positive electrode through an electrolyte and back to the negative electrode upon charging. Most commercial rechargable Li-ion batteries use a graphite anode (negative electrode) and a LiCoO2 cathode (positive electrode) in a design pioneered by Sony.
Check out these links for more info!
- Origin of Enhancements Using RGO - A webinar on the enhancements in battery operation by utilizing reduced graphene oxide as an electrode substrate.
- Is Lithium-ion the Ideal Battery? - Learn about some of the fundamental advantages and disadvantages of Li-ion battery technology from the Battery University.
Our Research Focus
- Improve Li-ion intercalation rates for faster charging/discharging
- Couple Li-ion cathodes with carbon based nanostructures for improved Li-ion diffusion and charge transport
- Increase battery lifetime through the design of more stable cathode composites
- Elucidate the mechanistic aspects of common Li-ion battery materials
Recent Progress
- Doped MnO2 nanowires on graphene oxide with nickel for a stable Li-ion battery material
- Synthesized "holey" graphene which may increase diffusion and lead to better Li-ion batteries
- Implemented reduced graphene oxide (RGO) into the anode of an α-MnO2 based Li-ion battery that showed a capacity of ∼150mAh/g after 20 cycles at 0.4C rate
- Elucidated the mechanistic nature of the performance increases seen in Li-ion batteries of the RGO on the commonly observed improvements in cycling and capacity
Select Publications
444. Nickel-Doped MnO2 Nanowires Anchored onto Reduced Graphene Oxide for Rapid Cycling Cathode in Lithium Ion Batteries Radich, J. G.; Chen, Y.-S.; Kamat, P. V. ECS Journal of Solid State Science and Technology 2013, 2 (10), M3178-M3181.
439. Making Graphene Holey. Gold-Nanoparticle-Mediated Hydroxyl Radical Attack on Reduced Graphene Oxide Radich, J. G.; Kamat, P. V. ACS Nano 2013, 7 (6), 5546–5557.
431. Graphitic Design: Prospects of Graphene-Based Nanocomposites for Solar Energy Conversion, Storage, and Sensing. Lightcap, I. V.; Kamat, P. V. Acc. Chem. Res. 2013, 46 (10), 2235–2243.
425. Origin of Reduced Graphene Oxide Enhancements in Electrochemical Energy Storage. Radich, J. G.; Kamat, P. V. ACS Catal. 2012, 2, 807–816.
407. Graphene-based Composites for Electrochemical Energy Storage Radich, J. G.; McGinn, P. J.; Kamat, P. V. Interface 2011, Spring Issue, 63-66.
404. Graphene-based Nanoassemblies for Energy Conversion Kamat, P. V. J. Phys. Chem. Lett. 2011, 2, 242-251. (Perspective article)
